MartynosideCAS# 67884-12-2 |
Quality Control & MSDS
Number of papers citing our products

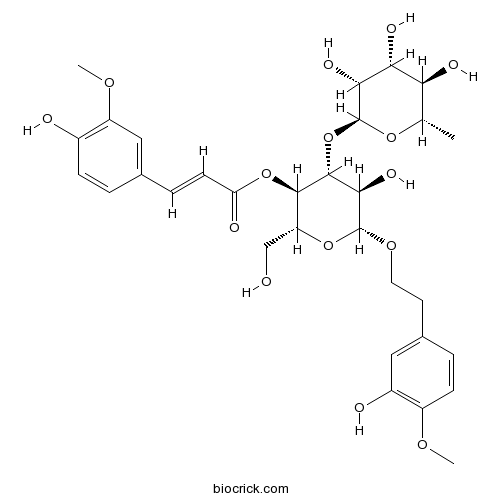
Chemical structure

3D structure
| Cas No. | 67884-12-2 | SDF | Download SDF |
| PubChem ID | 5319292 | Appearance | Powder |
| Formula | C31H40O15 | M.Wt | 652.7 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2R,3R,4R,5R,6R)-5-hydroxy-6-[2-(3-hydroxy-4-methoxyphenyl)ethoxy]-2-(hydroxymethyl)-4-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-3-yl] (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(OC(C2OC(=O)C=CC3=CC(=C(C=C3)O)OC)CO)OCCC4=CC(=C(C=C4)OC)O)O)O)O)O | ||
| Standard InChIKey | WLWAYPFRKDSFCL-CNMJWYMJSA-N | ||
| Standard InChI | InChI=1S/C31H40O15/c1-15-24(36)25(37)26(38)31(43-15)46-29-27(39)30(42-11-10-17-5-8-20(40-2)19(34)12-17)44-22(14-32)28(29)45-23(35)9-6-16-4-7-18(33)21(13-16)41-3/h4-9,12-13,15,22,24-34,36-39H,10-11,14H2,1-3H3/b9-6+/t15-,22+,24-,25+,26+,27+,28+,29+,30+,31-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Martynoside has antioxidative activity. 2. Martynoside is a natural selective estrogen receptor modulator. 3. Martynoside has anticancer and antimetastatic activities. 4. Martynoside has the potential of antagonizing sports anaemia, the mechanism of this effect might be related to preventing RBC from free radical damage. 5. Martynoside and verbascoside can resist muscle fatigue, which is depending on their antioxidative activities. |
| Targets | Estrogen receptor | ROS | Progestogen receptor |

Martynoside Dilution Calculator

Martynoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5321 mL | 7.6605 mL | 15.321 mL | 30.6419 mL | 38.3024 mL |
| 5 mM | 0.3064 mL | 1.5321 mL | 3.0642 mL | 6.1284 mL | 7.6605 mL |
| 10 mM | 0.1532 mL | 0.766 mL | 1.5321 mL | 3.0642 mL | 3.8302 mL |
| 50 mM | 0.0306 mL | 0.1532 mL | 0.3064 mL | 0.6128 mL | 0.766 mL |
| 100 mM | 0.0153 mL | 0.0766 mL | 0.1532 mL | 0.3064 mL | 0.383 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Deapi-platycodin D3
Catalog No.:BCN3240
CAS No.:67884-05-3
- Glycosolone
Catalog No.:BCN6521
CAS No.:67879-81-6
- Caftaric acid
Catalog No.:BCN2096
CAS No.:67879-58-7
- Boc-Nva-OH.DCHA
Catalog No.:BCC2642
CAS No.:67861-96-5
- Taraxasterone
Catalog No.:BCN7746
CAS No.:6786-16-9
- Ethyl 2,4-dihydroxyphenylacetate
Catalog No.:BCN4235
CAS No.:67828-62-0
- Methyl 2,4-dihydroxyphenylacetate
Catalog No.:BCN6801
CAS No.:67828-42-6
- 2,3,2'',3''-Tetrahydroochnaflavone
Catalog No.:BCN4234
CAS No.:678138-59-5
- MRK 560
Catalog No.:BCC2345
CAS No.:677772-84-8
- Menisdaurin
Catalog No.:BCN2552
CAS No.:67765-58-6
- 2-(2,4-Dihydroxyphenyl)-6-hydroxybenzofuran
Catalog No.:BCN7546
CAS No.:67736-22-5
- PIK-90
Catalog No.:BCC1248
CAS No.:677338-12-4
- n-Butyl-β-D-fructopyranoside
Catalog No.:BCC9097
CAS No.:67884-27-9
- Gigantol
Catalog No.:BCN8382
CAS No.:67884-30-4
- Cyclo(Pro-Trp)
Catalog No.:BCN2422
CAS No.:67889-75-2
- Scutebarbatine K
Catalog No.:BCN3223
CAS No.:960302-86-7
- Ambrox
Catalog No.:BCN6907
CAS No.:6790-58-5
- Aluminum n-octacosoxide
Catalog No.:BCC8099
CAS No.:67905-27-5
- Dehydroevodiamine
Catalog No.:BCN2974
CAS No.:67909-49-3
- Homobaldrinal
Catalog No.:BCN2681
CAS No.:67910-07-0
- Macusine B
Catalog No.:BCN6471
CAS No.:6792-07-0
- Mesaconine
Catalog No.:BCC8339
CAS No.:6792-09-2
- 1,6-Dihydro-4,7'-epoxy-1-methoxy-3',4'-methylenedioxy-6-oxo-3,8'-lignan
Catalog No.:BCN6584
CAS No.:67920-48-3
- Sodium Danshensu
Catalog No.:BCN5952
CAS No.:67920-52-9
Martynoside and the Novel Dimeric Open-Chain Monoterpene Glucoside Digipenstroside from Penstemon digitalis.[Pubmed:17262462]
Planta Med. 1989 Oct;55(5):474-6.
From the leaves of PENSTEMON DIGITALIS Nutt. a novel dimeric open-chain monoterpene glucoside, digipenstroside, in addition to the known phenylpropanoid glycoside Martynoside has been isolated. The structure of digipenstroside was elucidated by spectroscopic means (FD-MS, (1)H-, (13)C-, and 2D-NMR spectroscopy) as 1-(beta- D-glucopyranosyl)-8-[8''-hydroxy-2''-6''-dimethyl-oct-2''( E),6''( E)-dienoyl]-5,8-dihydroxy-2,6-dimethylocta-2( E),6( E)-dienoate. Therefore digipenstroside belongs to a new type of natural compounds, comprising of two geraniol-type monoterpenes and glucose linked by esterification. The occurrence of such compounds together with intact iridoid glycosides might be of interest from the biogenetic point of view.
Antioxidative properties of Martynoside: pulse radiolysis and laser photolysis study.[Pubmed:14567442]
Free Radic Res. 2003 Aug;37(8):829-33.
Free radical reactions of Martynoside (MAR), a phenylpropanoid glycoside, with a variety of oxidants were studied in the aqueous solution by laser photolysis and pulse radiolysis techniques. The pKa value of MAR in aqueous solution was measured from the pH dependent changes of the UV absorption at 384 nm with value of pKa = 9.2. The phenoxyl radical of MAR which exhibits maximum absorption at 360 nm was generated by one-electron transfer to N3* or Br2*-. Other important properties of phenoxyl radical such as extinction coefficient, formation and decay rate constants were also determined. The reaction rate constant of O2*- with MAR, k = 8.5 x 10(4) dm3 x mol(-1) x s(-1), was measured by the method of competition kinetics. By measuring time-resolved luminescence emission at 1270 nm, the quenching rate constant of singlet oxygen by MAR was obtained to be 3.3 x 10(6) dm3 x mol(-1) x s(-1). Reduction potential of the MAR couple (MAR*/MAR), determined using rutin as reference compound, gave a value E = 0.66 V vs. NHE. The antioxidative properties of MAR were compared with those of some well-known antioxidants.
Acteoside and martynoside exhibit estrogenic/antiestrogenic properties.[Pubmed:16198557]
J Steroid Biochem Mol Biol. 2006 Jan;98(1):63-71.
Acteoside and Martynoside are plant phenylpropanoid glycosides exhibiting anticancer, cytotoxic and antimetastatic activities. We investigated their potential to activate estrogen receptor isoforms ERalpha and ERbeta in HeLa cells transfected with an estrogen response element (ERE)-driven luciferase (Luc) reporter gene and an ERalpha or ERbeta expression vector. Their estrogenic/antiestrogenic effects were also assessed in breast cancer cells (MCF7), endometrial cancer cells (Ishikawa) and osteoblasts (KS483), by measuring IGFBP3 levels, cell viability and number of mineralized nodules, respectively, seeking for a natural selective estrogen receptor modulator (SERM). Acteoside and Martynoside antagonized both ERalpha and ERbeta (p<0.001), whereas they reversed the effect of E(2) mainly via ERalpha (p<0.001). Martynoside was a potent antiestrogen in MCF-7 cells, increasing, like ICI182780, IGFBP3 levels via the ER-pathway. In osteoblasts, Martynoside induced nodule mineralization, which was abolished by ICI182780, implicating an ER-mediated mechanism. Furthermore, its antiproliferative effect on endometrial cells suggests that Martynoside may be an important natural SERM. Acteoside was an antiestrogen in breast cancer cells and osteoblasts, without any effect on endometrial cells. Our study suggests that the nature is rich in selective ERalpha and ERbeta ligands, the discovery of which may lead to the development of novel neutraceutical agents.
Retardation of skeletal muscle fatigue by the two phenylpropanoid glycosides: verbascoside and martynoside from Pedicularis plicata maxim.[Pubmed:10548760]
Phytother Res. 1999 Nov;13(7):621-3.
The effects of the phenylpropanoid glycosides verbascoside and Martynoside from Pedicularis plicata were investigated on muscle contractility in Bufo gastrocnemius muscle electrically stimulated in vitro. The maximum amplitude and maintained time of contraction were mechanically recorded and used as indices of muscle contractility. After 30 min pretreatment of the muscle, verbascoside at 20.0 microM resisted muscle fatigue significantly while Martynoside at 80.0 microM improved muscle contractility only slightly. These two glycosides resisted muscle fatigue depending on their antioxidative activities, which is in agreement with the role of reactive oxygen species (ROS) in promoting fatigue in skeletal muscle.
Anti-sports anaemia effects of verbascoside and martynoside in mice.[Pubmed:20556696]
Int J Sports Med. 2010 Aug;31(8):537-41.
This paper aims to investigate the effects of verbascoside and Martynoside isolated from PEDICULARIS DOLICHOCYMBA on sports anaemia. Forty mice were divided into four groups: Group R (control group, nonsupplemented and maintained at rest), Group E (nonsupplemented and undergoing exercise), Group VE (supplemented with verbascoside 10 mg/kg per day and undergoing exercise), and Group ME (supplemented with Martynoside 10 mg/kg per day and undergoing exercise). After 5 weeks intensive swimming exercises, we measured the RBC count, the hemoglobin concentration, the hematocrit (Hct), the mean corpuscular hemoglobin concentration (MCHC) and the mean corpuscular hemoglobin (MCH). We studied the shapes of RBC and measured the plasma malonyldialdehyde (MDA). We found Group E showed lower RBC, hemoglobin and Hct levels, higher MCHC, MCH, plasma MDA levels and the abnormally shaped RBCs percentage than Groups R, VE and ME. Group ME showed lower RBC and Hct levels, higher MCH, plasma MDA levels and the abnormally shaped RBCs percentage than Group VE. The results indicated that verbascoside and Martynoside have the potential of antagonizing sports anaemia, the mechanism of this effect might be related to preventing RBC from free radical damage. Moreover, verbascoside was found to be more active than Martynoside.


