Loteprednol etabonateCAS# 82034-46-6 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Rosuvastatin Calcium
Catalog No.:BCC3853
CAS No.:147098-20-2
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 82034-46-6 | SDF | Download SDF |
| PubChem ID | 444025 | Appearance | Powder |
| Formula | C24H31ClO7 | M.Wt | 466.95 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 45 mg/mL (96.37 mM) *"≥" means soluble, but saturation unknown. | ||
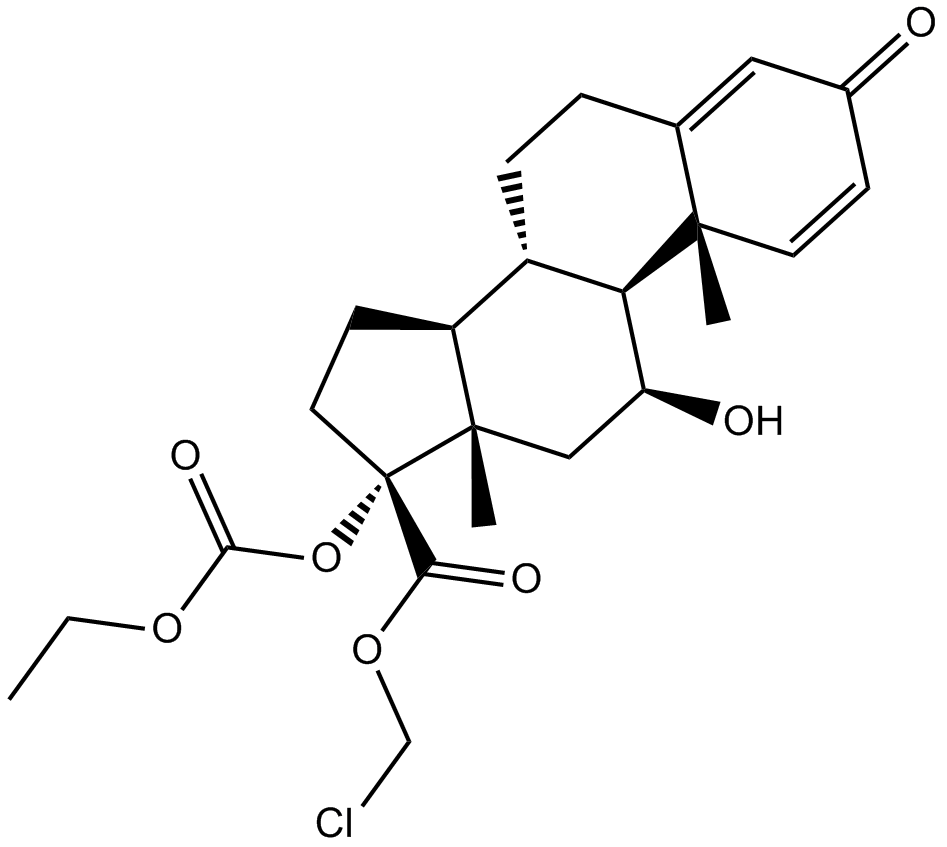
| Chemical Name | chloromethyl (8S,9S,10R,11S,13S,14S,17R)-17-ethoxycarbonyloxy-11-hydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-17-carboxylate | ||
| SMILES | CCOC(=O)OC1(CCC2C1(CC(C3C2CCC4=CC(=O)C=CC34C)O)C)C(=O)OCCl | ||
| Standard InChIKey | DMKSVUSAATWOCU-HROMYWEYSA-N | ||
| Standard InChI | InChI=1S/C24H31ClO7/c1-4-30-21(29)32-24(20(28)31-13-25)10-8-17-16-6-5-14-11-15(26)7-9-22(14,2)19(16)18(27)12-23(17,24)3/h7,9,11,16-19,27H,4-6,8,10,12-13H2,1-3H3/t16-,17-,18-,19+,22-,23-,24-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Loteprednol etabonate Dilution Calculator

Loteprednol etabonate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1416 mL | 10.7078 mL | 21.4156 mL | 42.8311 mL | 53.5389 mL |
| 5 mM | 0.4283 mL | 2.1416 mL | 4.2831 mL | 8.5662 mL | 10.7078 mL |
| 10 mM | 0.2142 mL | 1.0708 mL | 2.1416 mL | 4.2831 mL | 5.3539 mL |
| 50 mM | 0.0428 mL | 0.2142 mL | 0.4283 mL | 0.8566 mL | 1.0708 mL |
| 100 mM | 0.0214 mL | 0.1071 mL | 0.2142 mL | 0.4283 mL | 0.5354 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Loteprednol Etabonate is an anti-inflammatory corticosteroid used in optometry and ophthalmology.
- Boc-Leucinol
Catalog No.:BCC2724
CAS No.:82010-31-9
- 10Z-Hymenialdisine
Catalog No.:BCC5773
CAS No.:82005-12-7
- Daurichromenic acid
Catalog No.:BCN4355
CAS No.:82003-90-5
- 8-Anilino-1-naphthalenesulfonic acid
Catalog No.:BCC8785
CAS No.:82-76-8
- Peri acid
Catalog No.:BCC9116
CAS No.:82-75-7
- Visnagin
Catalog No.:BCN4367
CAS No.:82-57-5
- 1-Amino-2-methylanthraquinone
Catalog No.:BCC8451
CAS No.:82-28-0
- Alpha-Toxicarol
Catalog No.:BCN6467
CAS No.:82-09-7
- Rottlerin
Catalog No.:BCC7127
CAS No.:82-08-6
- Benzanthrone
Catalog No.:BCC8845
CAS No.:82-05-3
- Khellin
Catalog No.:BCN4356
CAS No.:82-02-0
- Lancerin
Catalog No.:BCN2803
CAS No.:81991-99-3
- NSC 33994
Catalog No.:BCC2441
CAS No.:82058-16-0
- JNJ 17203212
Catalog No.:BCC7668
CAS No.:821768-06-3
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
- FMK
Catalog No.:BCC1580
CAS No.:821794-92-7
- Andropanoside
Catalog No.:BCN4570
CAS No.:82209-72-1
- Andrographiside
Catalog No.:BCN4569
CAS No.:82209-76-5
- Atomoxetine HCl
Catalog No.:BCC5046
CAS No.:82248-59-7
- Impurity C of Alfacalcidol
Catalog No.:BCC5385
CAS No.:82266-85-1
- Breyniaionoside A
Catalog No.:BCN7112
CAS No.:823182-23-6
- Styraxlignolide F
Catalog No.:BCN3416
CAS No.:823214-06-8
- SN 2
Catalog No.:BCC6325
CAS No.:823218-99-1
- Coelonin
Catalog No.:BCN3600
CAS No.:82344-82-9
Comparison of the Safety and Efficacy of Loteprednol Etabonate 0.5%/Tobramycin 0.3% with Dexamethasone 0.1%/Tobramycin 0.3% Following Strabismus Surgery.[Pubmed:28149143]
Eurasian J Med. 2016 Oct;48(3):186-188.
OBJECTIVE: To compare the anti-inflammatory efficacy and safety of 0.5% Loteprednol etabonate/0.3% tobramycin (LE/T) and 0.1% dexamethasone/0.3% tobramycin (DM/T) ophthalmic suspensions following strabismus surgery. MATERIALS AND METHODS: The records of 40 patients who were treated with either LE/T or DM/T following strabismus surgery were retrospectively reviewed. The recorded signs and symptoms of inflammation and intraocular pressure of the patients at 1 day, 1 week, and 3 weeks after the surgery were evaluated and compared between the groups. RESULTS: In both groups, reduced inflammation was noted during the follow-up visits. There was no statistically significant difference between the LE/T and DM/T groups with regard to the postoperative scores or measurements, including discomfort, chemosis, secretion, conjunctival hyperemia, and conjunctival gap size (p>0.05), during the follow-up visits. Allergic reactions to the medications were not reported in any patient. Intraocular pressures were within normal limits in both groups. CONCLUSION: LE/T was found to be as effective as DM/T in reducing inflammation after strabismus surgery. LE/T, as a new-generation steroid combination product, could be used as a safe and effective anti-inflammatory agent for the treatment of inflammation following strabismus surgery.
Development of loteprednol etabonate-loaded cationic nanoemulsified in-situ ophthalmic gel for sustained delivery and enhanced ocular bioavailability.[Pubmed:27689408]
Drug Deliv. 2016 Nov;23(9):3712-3723.
A novel cationic nanoemulsified in-situ ophthalmic gel of Loteprednol etabonate (LE) was developed to improve the permeability and retention time of formulations for overall improvement of drug's ocular bioavability. Capryol 90 (oil phase), tween 80 (surfactant) and transcutol P (cosurfactant) was selected as formulation excipients to construct pseudoternary phase diagrams and nanoemulsion region was recognized from diagrams. Spontaneous emulsification method was used to manufacture LE nanoemulsion and it was optimized using 3(2) factorial design by considering the amount of oil and the ratio of surfactant to cosurfactant (Smix) as independent variables and evaluated for various physicochemical properties. Optimized NE was dispersed in Poloxamer 407 and 188 solution to form nanoemulsified sols that were predictable to transform into in-situ gels at corneal temperature. Drug pharmacokinetics of sterilized optimized in situ NE gel, NE-ISG2 [0.69% w/w Capryol 90, 0.99%w/w Smix (3:1), 13% Poloxamer 407, 4% w/w Poloxamer 188] and marketed formulation were assessed in rabbit aqueous humor. The in-situ gels were clear, shear thinning in nature and displayed zero-order drug release kinetics. NE-ISG2 showed the minimum ocular irritation potential and significantly (p < 0.01) higher Cmax and AUC(0-10 h), delayed Tmax, extended mean residence time and improved (2.54-fold times) bioavailability compared to marketed formulation.
Dose Uniformity of Topical Corticosteroids: A Simulated Trial of Fluorometholone Acetate 0.1% and Loteprednol Etabonate Gel 0.5.[Pubmed:28140772]
J Ocul Pharmacol Ther. 2017 Mar;33(2):111-114.
PURPOSE: The purpose of the study was to determine the concentrations of Flarex((R)) and Lotemax((R)) when shaken and not shaken. Many patients fail to shake or inappropriately shake suspensions of corticosteroids before instillation as directed. This study was designed to help determine what concentration of corticosteroid these patients are receiving. In addition, independent confirmation of Loteprednol etabonate ophthalmic gel dose uniformity was determined and compared as a possible alternative. METHODS: Drug concentrations of shaken versus unshaken Flarex and Lotemax were determined over a 20-day simulated tapered course in our institutional laboratory. Collected samples were analyzed by reversed-phase high-performance liquid chromatography with photodiode array detection at 240 nm. RESULTS: Flarex had a mean concentration of 93.7% of the declared concentration when shaken and 7.25% when not shaken. The difference between these groups was statistically significant (P = 0.0001). Lotemax had a mean concentration of 96.74% of the declared concentration when shaken and a mean concentration of 98.97% when not shaken. The difference between these groups was not statistically significant (P = 0.194). CONCLUSIONS: Flarex maintains dose uniformity when shaken. When not shaken, it has poor dose uniformity. Lotemax was consistent whether shaken or not in our study and can be considered to eliminate the variability of poor patient compliance with shaking. The manufacturers of both drugs recommend shaking before application.
Efficacy of olopatadine hydrochloride 0.1%, emedastine difumarate 0.05%, and loteprednol etabonate 0.5% for Chinese children with seasonal allergic conjunctivitis: a randomized vehicle-controlled study.[Pubmed:27869354]
Int Forum Allergy Rhinol. 2017 Apr;7(4):393-398.
BACKGROUND: Allergic conjunctivitis (AC) is a disease of various agents that affects the physical and mental health of children. Although the most effective therapy has not been found so far, it is essential to explore the considerable therapeutic method. We compared the clinical efficacy of olopatadine, emedastine, Loteprednol etabonate (LE), and vehicle for treating seasonal allergic conjunctivitis (SAC) in Chinese children. METHODS: Eighty cases of 160 eyes aged from 5 to 10 years with SAC were available and those subjects were randomly distributed into 4 groups. Both their eyes received olopatadine hydrochloride 0.1% twice a day, emedastine difumarate 0.05% twice a day, or LE 0.5% 4 times a day, respectively, whereas those of the control group received artificial tears (AT) 0.5% 3 times a day. This study was conducted successfully and the observations were collected before treatment and on day 8 (+/-1 day) and day 15 (+/-2 days) afterward. The principal measurement of efficacy was focused on the signs and symptoms of the subjects, evaluated before and after treatment, in addition to visual acuity (VA) and fundus oculi. RESULTS: On day 8 (+/-1 day) and day 15 (+/-2 days), all the antiallergic agents were found to be more effective than vehicle (p < 0.05) in terms of all the symptoms and signs. However, there was no statistical significance (p >/= 0.05) shown among the treatment groups. There were no evident changes in VA and no clinically significant changes were observed in fundus oculi. CONCLUSION: After the treatment, the efficacy presented a similar distribution among the trial groups.


