LP 44High affinity 5-HT7 agonist CAS# 824958-12-5 |
- Atorvastatin Calcium
Catalog No.:BCC2319
CAS No.:134523-03-8
- Pitavastatin Calcium
Catalog No.:BCC3842
CAS No.:147526-32-7
- Pravastatin sodium
Catalog No.:BCC2321
CAS No.:81131-70-6
- Fluvastatin
Catalog No.:BCC1579
CAS No.:93957-54-1
- Fluvastatin Sodium
Catalog No.:BCC2317
CAS No.:93957-55-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 824958-12-5 | SDF | Download SDF |
| PubChem ID | 11225543 | Appearance | Powder |
| Formula | C27H38ClN3OS | M.Wt | 488.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 75 mM in ethanol | ||
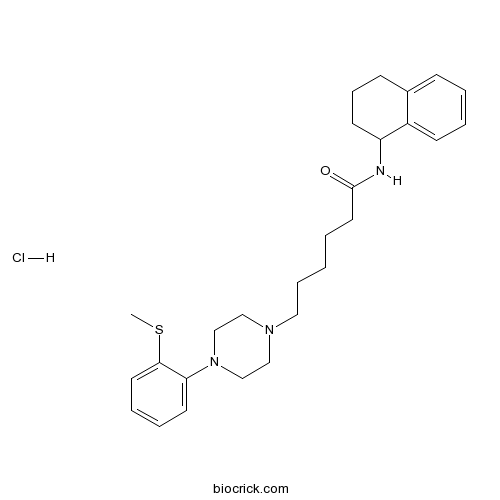
| Chemical Name | 6-[4-(2-methylsulfanylphenyl)piperazin-1-yl]-N-(1,2,3,4-tetrahydronaphthalen-1-yl)hexanamide;hydrochloride | ||
| SMILES | CSC1=CC=CC=C1N2CCN(CC2)CCCCCC(=O)NC3CCCC4=CC=CC=C34.Cl | ||
| Standard InChIKey | DWGKCWWWKHCVDH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H37N3OS.ClH/c1-32-26-15-7-6-14-25(26)30-20-18-29(19-21-30)17-8-2-3-16-27(31)28-24-13-9-11-22-10-4-5-12-23(22)24;/h4-7,10,12,14-15,24H,2-3,8-9,11,13,16-21H2,1H3,(H,28,31);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity 5-HT7 receptor agonist (Ki = 0.22 nM) that displays selectivity over 5-HT1A and 5-HT2A receptors (200- and > 1000-fold respectively). Induces relaxation of substance P-stimulated guinea pig ileum (EC50 = 2.56 μM). |

LP 44 Dilution Calculator

LP 44 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0486 mL | 10.2432 mL | 20.4863 mL | 40.9727 mL | 51.2159 mL |
| 5 mM | 0.4097 mL | 2.0486 mL | 4.0973 mL | 8.1945 mL | 10.2432 mL |
| 10 mM | 0.2049 mL | 1.0243 mL | 2.0486 mL | 4.0973 mL | 5.1216 mL |
| 50 mM | 0.041 mL | 0.2049 mL | 0.4097 mL | 0.8195 mL | 1.0243 mL |
| 100 mM | 0.0205 mL | 0.1024 mL | 0.2049 mL | 0.4097 mL | 0.5122 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Baicalin methyl ester
Catalog No.:BCN3252
CAS No.:82475-03-4
- Oroxylin A 7-O-beta-D-glucuronide methyl ester
Catalog No.:BCN1339
CAS No.:82475-01-2
- Neokadsuranin
Catalog No.:BCN7816
CAS No.:115181-68-5
- R(+)-Gomisin M1
Catalog No.:BCN4362
CAS No.:82467-50-3
- 3-Oxotirucalla-7,24-dien-21-oic acid
Catalog No.:BCN4568
CAS No.:82464-35-5
- Hispanone
Catalog No.:BCN7404
CAS No.:82462-67-7
- Arteannuin A
Catalog No.:BCN4361
CAS No.:82442-48-6
- Pulchinenoside E3
Catalog No.:BCN8187
CAS No.:824401-07-2
- Agomelatine L(+)-Tartaric acid
Catalog No.:BCC4211
CAS No.:824393-18-2
- Maglifloenone
Catalog No.:BCN4360
CAS No.:82427-77-8
- Gomisin M2
Catalog No.:BCN4359
CAS No.:82425-45-4
- Gomisin L2
Catalog No.:BCN7032
CAS No.:82425-44-3
- A 784168
Catalog No.:BCC6140
CAS No.:824982-41-4
- Pseudolaric Acid B
Catalog No.:BCN6284
CAS No.:82508-31-4
- Pseudolaric acid A
Catalog No.:BCN3717
CAS No.:82508-32-5
- Methyl pseudolarate A
Catalog No.:BCN4363
CAS No.:82508-33-6
- Methyl pseudolarate B
Catalog No.:BCN4364
CAS No.:82508-34-7
- Demethoxydeacetoxypseudolaric acid B
Catalog No.:BCN4365
CAS No.:82508-36-9
- Deacetylpseudolaric acid A
Catalog No.:BCN4366
CAS No.:82508-37-0
- 10-Hydroxy-16-epiaffinine
Catalog No.:BCN4001
CAS No.:82513-70-0
- 5-Methyl-7-methoxyisoflavone
Catalog No.:BCN8465
CAS No.:82517-12-2
- Myoscorpine
Catalog No.:BCN1973
CAS No.:82535-76-0
- Fmoc-Phe(4-I)-OH
Catalog No.:BCC3261
CAS No.:82565-68-2
- Ozagrel
Catalog No.:BCC2298
CAS No.:82571-53-7
Selective 5-HT7 receptor agonists LP 44 and LP 211 elicit an analgesic effect on formalin-induced orofacial pain in mice.[Pubmed:27383702]
J Appl Oral Sci. 2016 May-Jun;24(3):218-22.
OBJECTIVE: To investigate the antinociceptive effects of pharmacological activation of 5-HT7 receptors on orofacial pain in mice. MATERIAL AND METHODS: Nociception was evaluated by using an orofacial formalin test in male Balb-C mice. Selective 5-HT7 receptor agonists, LP 44 and LP 211 (1, 5, and 10 mg/kg), were given intraperitoneally 30 min prior to a formalin injection. A bolus of 10 microl of 4% subcutaneous formalin was injected into the upper lip of mice and facial grooming behaviors were monitored. The behavioral responses consisted of two distinct periods, the early phase corresponding to acute pain (Phase I: 0-12 min) and the late phase (Phase II: 12-30 min). RESULTS: LP 44 and LP 211 (1, 5, and 10 mg/kg) produced an analgesic effect with reductions in face rubbing time in both Phase I and Phase II of the formalin test. CONCLUSION: Our results suggest that 5-HT7 receptor agonists may be promising analgesic drugs in the treatment of orofacial pain.
The serotonin 5-HT7 receptor agonist LP-44 microinjected into the dorsal raphe nucleus suppresses REM sleep in the rat.[Pubmed:18466985]
Behav Brain Res. 2008 Aug 22;191(2):184-9.
The effects of LP-44, a selective 5-HT7 receptor agonist, and of SB-269970, a selective 5-HT7 receptor antagonist, on spontaneous sleep were studied in adult rats implanted for chronic sleep recordings. The 5-HT7 receptor ligands were microinjected directly into the dorsal raphe nucleus (DRN) during the light period of the 12-h light/12-h dark cycle. Infusion of LP-44 (1.25-5.0 mM) into the DRN induced a significant reduction of rapid-eye-movement sleep (REMS) and of the number of REM periods. Similar effects were observed after the direct administration into the DRN of SB-269970 (0.5-1.0 mM). Pretreatment with a dose of SB-269970 (0.5 mM) that significantly affects sleep variables antagonized the LP-44 (2.5 mM)-induced suppression of REMS and of the number of REM periods. It is proposed that the suppression of REMS after microinjection of LP-44 into the DRN is related, at least in part, to the activation of GABAergic neurons in the DRN that contribute to long projections that reach, among others, the laterodorsal and pedunculopontine tegmental nuclei involved in the promotion of REMS.
Detection of Lead in the Carbon-rich, Very Metal-poor Star LP 625-44: A Strong Constraint on s-Process Nucleosynthesis at Low Metallicity.[Pubmed:10859127]
Astrophys J. 2000 Jun 20;536(2):L97-L100.
We report the detection of the Pb i lambda4057.8 line in the very metal-poor (&sqbl0;Fe&solm0;H&sqbr0;=-2.7), carbon-rich star, LP 625-44. We determine the abundance of Pb (&sqbl0;Pb&solm0;Fe&sqbr0;=2.65) and 15 other neutron-capture elements. The abundance pattern between Ba and Pb agrees well with a scaled solar system s-process component, while the lighter elements (Sr-Zr) are less abundant than Ba. The enhancement of s-process elements is interpreted as a result of mass transfer in a binary system from a previous asymptotic giant branch (AGB) companion, an interpretation strongly supported by radial velocity variations of this system. The detection of Pb makes it possible, for the first time, to compare model predictions of s-process nucleosynthesis in AGB stars with observations of elements between Sr and Pb. The Pb abundance is significantly lower than the prediction of recent models (e.g., Gallino et al.), which succeeded in explaining the metallicity dependence of the abundance ratios of light s-elements (Sr-Zr) to heavy ones (Ba-Dy) found in previously observed s-process-enhanced stars. This suggests that one should either (1) reconsider the underlying assumptions concerning the (13)C-rich s-processing site ((13)C pocket) in the present models or (2) investigate alternative sites of s-process nucleosynthesis in very metal-poor AGB stars.
Structure-affinity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinealkylamides, a new class of 5-hydroxytryptamine7 receptor agents.[Pubmed:15588097]
J Med Chem. 2004 Dec 16;47(26):6616-24.
A series of N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinealkylamides was prepared and their affinity for serotonin (5-hydroxytryptamine, 5-HT) 5-HT7, 5-HT(1A), and 5-HT(2A) receptors was measured by in vitro binding assays. In relation to 5-HT7 receptor affinity, receptor binding studies indicated that (i) the optimal alkyl chain length was five methylenes, (ii) an unsubstituted 1,2,3,4-tetrahydronaphthalenyl nucleus was preferred, and (iii) the substitution pattern of the aryl ring linked to the piperazine ring played a crucial role. Several compound with high affinity for 5-HT7 receptors were identified. Among them, 4-(2-methoxyphenyl)-N-(1,2,3,4-tetrahydronaphthalen-1-yl)-1-piperazinehexanamide (28), 4-(2-acetylphenyl)-N-(1,2,3,4-tetrahydronaphthalen-1-yl)-1-piperazinehexanamide (34), 4-(2-methylthiophenyl)-N-(1,2,3,4-tetrahydronaphthalen-1-yl)-1-piperazinehexanami de (44), 4-(2-hydroxyphenyl)-N-(1,2,3,4-tetrahydronaphthalen-1-yl)-1-piperazinehexanamide (46), and 4-(2-methylphenyl)-N-(1,2,3,4-tetrahydronaphthalen-1-yl)-1-piperazinehexanamide (49) were assayed for the 5-HT7 receptor-mediated relaxation of substance P-induced guinea pig ileum contraction. Compounds 28, 44, and 49 behaved as full agonists and compound 34 as a partial agonist, whereas derivative 46 acted as an antagonist. Among the compounds presented here, it emerged that 44 was identified as a potent 5-HT7 receptor agonist (Ki = 0.22 nM, EC50 = 2.56 microM), endowed with selectivity over 5-HT(1A) and 5-HT(2A) receptors (200-fold and >1000-fold, respectively).


