L-lysineCAS# 56-87-1 |
Quality Control & MSDS
Number of papers citing our products

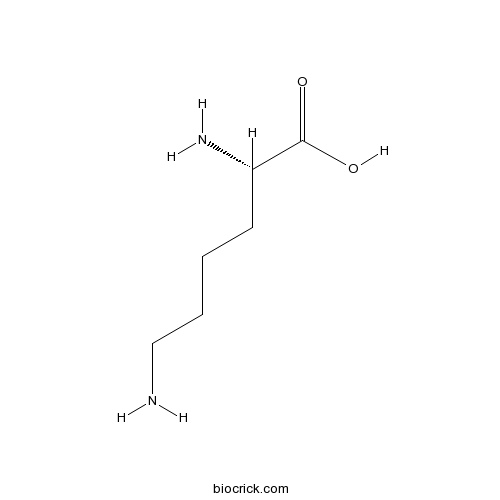
Chemical structure

3D structure
| Cas No. | 56-87-1 | SDF | Download SDF |
| PubChem ID | 5962 | Appearance | Powder |
| Formula | C6H14N2O2 | M.Wt | 146.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2,6-diaminohexanoic acid | ||
| SMILES | C(CCN)CC(C(=O)O)N | ||
| Standard InChIKey | KDXKERNSBIXSRK-YFKPBYRVSA-N | ||
| Standard InChI | InChI=1S/C6H14N2O2/c7-4-2-1-3-5(8)6(9)10/h5H,1-4,7-8H2,(H,9,10)/t5-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. L-lysine and L-glutamic acid and as useful building blocks for the preparation of bifunctional DTPA-like ligands. 2. Lysine is an essential amino acid for the human body. |

L-lysine Dilution Calculator

L-lysine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.8399 mL | 34.1997 mL | 68.3995 mL | 136.7989 mL | 170.9986 mL |
| 5 mM | 1.368 mL | 6.8399 mL | 13.6799 mL | 27.3598 mL | 34.1997 mL |
| 10 mM | 0.684 mL | 3.42 mL | 6.8399 mL | 13.6799 mL | 17.0999 mL |
| 50 mM | 0.1368 mL | 0.684 mL | 1.368 mL | 2.736 mL | 3.42 mL |
| 100 mM | 0.0684 mL | 0.342 mL | 0.684 mL | 1.368 mL | 1.71 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-Glutamic acid
Catalog No.:BCN3809
CAS No.:56-86-0
- L-Glutamine
Catalog No.:BCC3803
CAS No.:56-85-9
- H-Asp-OH
Catalog No.:BCC2881
CAS No.:56-84-8
- Glycerol
Catalog No.:BCC8990
CAS No.:56-81-5
- Chloramphenicol
Catalog No.:BCC1201
CAS No.:56-75-7
- DL-5-Hydroxytryptophan
Catalog No.:BCN1232
CAS No.:56-69-9
- Quinidine
Catalog No.:BCC7863
CAS No.:56-54-2
- Diethylstilbestrol
Catalog No.:BCC4900
CAS No.:56-53-1
- Deoxycorticosterone acetate
Catalog No.:BCC4655
CAS No.:56-47-3
- H-Ser-OH
Catalog No.:BCC3028
CAS No.:56-45-1
- H-Ala-OH
Catalog No.:BCC3190
CAS No.:56-41-7
- H-Gly-OH
Catalog No.:BCC2946
CAS No.:56-40-6
- (H-Cys-OH)2
Catalog No.:BCC2915
CAS No.:56-89-3
- Histamine 2HCl
Catalog No.:BCC4530
CAS No.:56-92-8
- Chlorhexidine acetate
Catalog No.:BCC8912
CAS No.:56-95-1
- 9-Hydroxy-4-androstene-3,17-dione
Catalog No.:BCC8802
CAS No.:560-62-3
- Eburicoic acid
Catalog No.:BCN2556
CAS No.:560-66-7
- Sucralose
Catalog No.:BCC4725
CAS No.:56038-13-2
- Hispidin
Catalog No.:BCN3567
CAS No.:56070-89-4
- PRIMA-1
Catalog No.:BCC2413
CAS No.:5608-24-2
- 3-Methoxyshancigusin I
Catalog No.:BCC9002
CAS No.:
- Ionomycin free acid
Catalog No.:BCC7261
CAS No.:56092-81-0
- Ionomycin calcium salt
Catalog No.:BCC5805
CAS No.:56092-82-1
- Securinine
Catalog No.:BCN6988
CAS No.:5610-40-2
Improved L-lysine yield with Corynebacterium glutamicum: use of dapA resulting in increased flux combined with growth limitation.[Pubmed:9487706]
Appl Microbiol Biotechnol. 1998 Jan;49(1):24-30.
The amino acid L-lysine is produced on a large scale using mutants of Corynebacterium glutamicum. However, as yet recombinant DNA techniques have not succeed in improving strains selected for decades by classic mutagenesis for high productivity. We here report that seven biosynthetic enzymes were assayed and oversynthesis of the dihydrodipicolinate synthase resulted in an increase of lysine accumulation from 220 mM to 270 mM. The synthase, encoded by dapA, is located at the branch point of metabolite distribution to either lysine or threonine and competes with homoserine dehydrogenase for the common substrate aspartate semialdehyde. When graded dapA expression was used, as well as quantification of enzyme activities, intracellular metabolite concentrations and flux rates, a global response of the carbon metabolism to the synthase activity became apparent: the increased flux towards lysine was accompanied by a decreased flux towards threonine. This resulted in a decreased growth rate, but increased intracellular levels of pyruvate-derived valine and alanine. Therefore, modulating the flux at the branch point results in an intrinsically introduced growth limitation with increased intracellular precursor supply for lysine synthesis. This does not only achieve an increase in lysine yield but this example of an intracellularly introduced growth limitation is proposed as a new general means of increasing flux for industrial metabolite over-production.
L-Glutamic acid and L-lysine as useful building blocks for the preparation of bifunctional DTPA-like ligands.[Pubmed:9893975]
Bioconjug Chem. 1999 Jan-Feb;10(1):137-40.
Bisalkylation of suitably protected L-glutamic acid and L-lysine derivatives with tert-butyl N-(2-bromoethyl)iminodiacetate 2, followed by deprotection of the omega functional group affords N, N-bis[2-[bis[2-(1, 1-dimethylethoxy)-2-oxoethyl]amino]ethyl]-L-glutamic acid 1-(1, 1-dimethylethyl) ester 4 and N2,N2-bis[2-[bis[2-(1, 1-dimethylethoxy)-2-oxoethyl]amino]ethyl]-L-lysine 1,1-dimethylethyl ester 7. Such compounds feature a carboxylic or an amino group, respectively, which are available for conjugation with a suitable partner via formation of an amide bond. The conjugates, which can be prepared in this way, contain a chelating subunit in which all five acetic residues of DTPA are available for the complexation of metal ions. Direct bisalkylation of glycine with 2 promptly gives N, N-bis[2-[bis[2-(1,1-dimethylethoxy)-2-oxoethyl]amino]ethyl]glycine 11. The latter allows to achieve conjugates in which the central acetic group of DTPA is selectively converted into an acetamide.
A genome-based approach to create a minimally mutated Corynebacterium glutamicum strain for efficient L-lysine production.[Pubmed:16506038]
J Ind Microbiol Biotechnol. 2006 Jul;33(7):610-5.
Based on the progress in genomics, we have developed a novel approach that employs genomic information to generate an efficient amino acid producer. A comparative genomic analysis of an industrial L-lysine producer with its natural ancestor identified a variety of mutations in genes associated with L-lysine biosynthesis. Among these mutations, we identified two mutations in the relevant terminal pathways as key mutations for L-lysine production, and three mutations in central metabolism that resulted in increased titers. These five mutations when assembled in the wild-type genome led to a significant increase in both the rate of production and final L-lysine titer. Further investigations incorporated with transcriptome analysis suggested that other as yet unidentified mutations are necessary to support the L-lysine titers observed by the original production strain. Here we describe the essence of our approach for strain reconstruction, and also discuss mechanisms of L-lysine hyperproduction unraveled by combining genomics with classical strain improvement.


