K 579Dipeptidyl peptidase IV inhibitor CAS# 440100-64-1 |
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- GF 109203X
Catalog No.:BCC3704
CAS No.:133052-90-1
- Go 6983
Catalog No.:BCC3705
CAS No.:133053-19-7
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
Number of papers citing our products

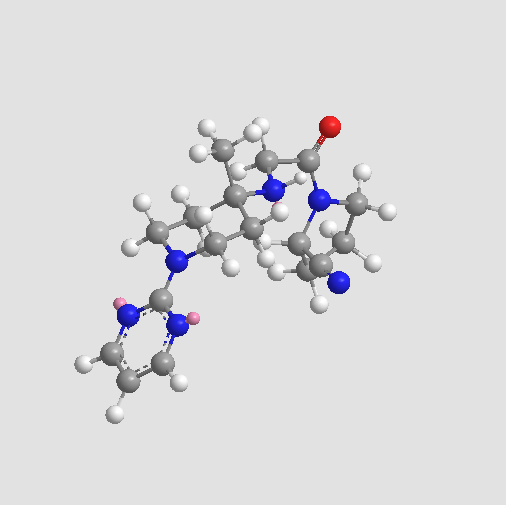
Chemical structure
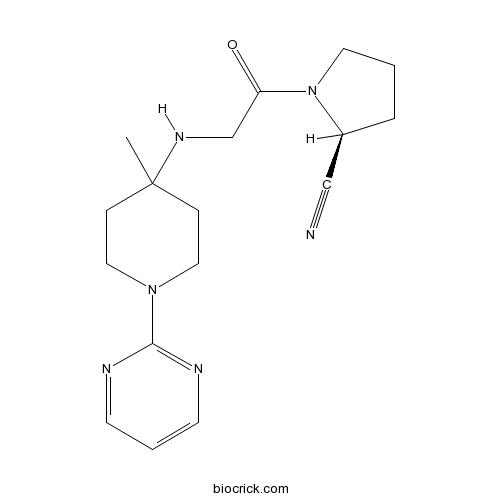
3D structure
| Cas No. | 440100-64-1 | SDF | Download SDF |
| PubChem ID | 9818824 | Appearance | Powder |
| Formula | C17H24N6O | M.Wt | 328.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in 1eq. HCl and to 100 mM in DMSO | ||
| Chemical Name | (2S)-1-[2-[(4-methyl-1-pyrimidin-2-ylpiperidin-4-yl)amino]acetyl]pyrrolidine-2-carbonitrile | ||
| SMILES | CC1(CCN(CC1)C2=NC=CC=N2)NCC(=O)N3CCCC3C#N | ||
| Standard InChIKey | JERPHRNGJBSFAP-AWEZNQCLSA-N | ||
| Standard InChI | InChI=1S/C17H24N6O/c1-17(21-13-15(24)23-9-2-4-14(23)12-18)5-10-22(11-6-17)16-19-7-3-8-20-16/h3,7-8,14,21H,2,4-6,9-11,13H2,1H3/t14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dipeptidyl peptidase IV inhibitor. Inhibits rat, canine, human and monkey enzymes with IC50 values of 3, 5, 8, and 8 nM respectively. Long-acting hypoglycemic agent; attenuates glucose excursion following glucose loading in Zucker fatty rats. |

K 579 Dilution Calculator

K 579 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.045 mL | 15.2249 mL | 30.4497 mL | 60.8995 mL | 76.1244 mL |
| 5 mM | 0.609 mL | 3.045 mL | 6.0899 mL | 12.1799 mL | 15.2249 mL |
| 10 mM | 0.3045 mL | 1.5225 mL | 3.045 mL | 6.0899 mL | 7.6124 mL |
| 50 mM | 0.0609 mL | 0.3045 mL | 0.609 mL | 1.218 mL | 1.5225 mL |
| 100 mM | 0.0304 mL | 0.1522 mL | 0.3045 mL | 0.609 mL | 0.7612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dipeptidyl peptidase IV inhibitor. Inhibits rat, canine, human and monkey enzymes with IC50 values of 3, 5, 8, and 8 nM respectively. Long-acting hypoglycemic agent; attenuates glucose excursion following glucose loading in Zucker fatty rats.
- Trifluoperazine 2HCl
Catalog No.:BCC4384
CAS No.:440-17-5
- Isoscabertopin
Catalog No.:BCN4634
CAS No.:439923-16-7
- Gnetifolin M
Catalog No.:BCN3394
CAS No.:439900-84-2
- NPY 5RA972
Catalog No.:BCC7747
CAS No.:439861-56-0
- BMS-509744
Catalog No.:BCC1424
CAS No.:439575-02-7
- ITK inhibitor
Catalog No.:BCC1662
CAS No.:439574-61-5
- GW 627368
Catalog No.:BCC7961
CAS No.:439288-66-1
- Lasmiditan
Catalog No.:BCC4077
CAS No.:439239-90-4
- Bay 60-7550
Catalog No.:BCC1405
CAS No.:439083-90-6
- Afatinib
Catalog No.:BCC3656
CAS No.:439081-18-2
- O-2093
Catalog No.:BCC7070
CAS No.:439080-01-0
- Gentianine
Catalog No.:BCN5492
CAS No.:439-89-4
- WAY 200070
Catalog No.:BCC7669
CAS No.:440122-66-7
- 3-Phenyl-2-propen-1-ol
Catalog No.:BCN5493
CAS No.:4407-36-7
- Benzoin oxime
Catalog No.:BCC8858
CAS No.:441-38-3
- Eribulin mesylate
Catalog No.:BCC5173
CAS No.:441045-17-6
- 2-(Cyanomethyl)benzimidazole
Catalog No.:BCC8483
CAS No.:4414-88-4
- 6-Acetylacteoside
Catalog No.:BCC8109
CAS No.:441769-43-3
- Macitentan
Catalog No.:BCC1142
CAS No.:441798-33-0
- Harmine
Catalog No.:BCN5494
CAS No.:442-51-3
- Clemizole
Catalog No.:BCC1485
CAS No.:442-52-4
- Aspertine C
Catalog No.:BCN2153
CAS No.:442155-62-6
- CK-636
Catalog No.:BCC5107
CAS No.:442632-72-6
- CK 666
Catalog No.:BCC6088
CAS No.:442633-00-3
The dipeptidyl peptidase IV inhibitors vildagliptin and K-579 inhibit a phospholipase C: a case of promiscuous scaffolds in proteins.[Pubmed:25671081]
F1000Res. 2013 Dec 27;2:286.
The long term side effects of any newly introduced drug is a subject of intense research, and often raging controversies. One such example is the dipeptidyl peptidase-IV (DPP4) inhibitor used for treating type 2 diabetes, which is inconclusively implicated in increased susceptibility to acute pancreatitis. Previously, based on a computational analysis of the spatial and electrostatic properties of active site residues, we have demonstrated that phosphoinositide-specific phospholipase C (PI-PLC) from Bacillus cereus is a prolyl peptidase using in vivo experiments. In the current work, we first report the inhibition of the native activity of PI-PLC by two DPP4 inhibitors - vildagliptin (LAF-237) and K-579. While vildagliptin inhibited PI-PLC at micromolar concentrations, K-579 was a potent inhibitor even at nanomolar concentrations. Subsequently, we queried a comprehensive, non-redundant set of 5000 human proteins (50% similarity cutoff) with known structures using serine protease (SPASE) motifs derived from trypsin and DPP4. A pancreatic lipase and a gastric lipase are among the proteins that are identified as proteins having promiscuous SPASE scaffolds that could interact with DPP4 inhibitors. The presence of such scaffolds in human lipases is expected since they share the same catalytic mechanism with PI-PLC. However our methodology also detects other proteins, often with a completely different enzymatic mechanism, that have significantly congruent domains with the SPASE motifs. The reported elevated levels of serum lipase, although contested, could be rationalized by inhibition of lipases reported here. In an effort to further our understanding of the spatial and electrostatic basis of DPP4 inhibitors, we have also done a comprehensive analysis of all 76 known DPP4 structures liganded to inhibitors till date. Also, the methodology presented here can be easily adopted for other drugs, and provide the first line of filtering in the identification of pathways that might be inadvertently affected due to promiscuous scaffolds in proteins.
K579, a slow-binding inhibitor of dipeptidyl peptidase IV, is a long-acting hypoglycemic agent.[Pubmed:14985056]
Eur J Pharmacol. 2004 Feb 23;486(3):335-42.
Dipeptidyl peptidase IV inhibitors are expected to be categorized in a new type of antidiabetic drugs. We had developed a long-acting dipeptidyl peptidase IV inhibitor, K579 [(S)-1-[4-methyl-1-(2-pyrimidinyl)-4-piperidylamino]acetyl-2-pyrrolidinecarbonitr ile]. The aim of present study was to characterize the pharmacological profiles of K579. In normal rats, K579 suppressed the blood glucose elevation after an oral glucose tolerance test with the increment of plasma insulin and active forms of glucagon-like peptide-1 (GLP-1). During repetitive glucose loading using Zucker fatty rats, pretreatment with K579 attenuated the glucose excursion after the second glucose loading as well as the first glucose loading without inducing hypoglycemia. The kinetic study using cell extract revealed that K579 was a more potent and slower binding inhibitor than the existing dipeptidyl peptidase IV inhibitor (NVP-DPP728, 1-[[[2-[(5-cyanopyridin-2-yl)amino]ethyl]amino]acetyl]-2-cyano-(S)-pyrrolidine). These profiles of K579 might be advantageous over the existing dipeptidyl peptidase IV inhibitor with respect to less dosing frequency.
Effects of combination treatment with dipeptidyl peptidase IV inhibitor and sulfonylurea on glucose levels in rats.[Pubmed:15215655]
J Pharmacol Sci. 2004 Jun;95(2):291-3.
The effects of orally administered dipeptidyl peptidase IV (DPP-IV) inhibitor on the glucose-lowering effect of glibenclamide are still unknown. We evaluated the effects of combination treatment with a long-lasting DPP-IV inhibitor, K579 ((S)-1-[4-methyl-1-(2-pyrimidinyl)-4-piperidylamino]acetyl-2-pyrrolidinecarbonitr ile), and glibenclamide on the glycemic responses to glucose loading in rats. Treatment with K579 inhibited the plasma DPP-IV activity even 8 h after the administration. K579 significantly suppressed the blood glucose elevation in glibenclamide-pretreated rats without excessive hypoglycemia. These profiles of K579 indicate that it could be useful agent to correct the postprandial glucose excursion in type 2 diabetes patients by combination treatment with glibenclamide.
Involvement of the active metabolites in the inhibitory activity of K579 on rat plasma dipeptidyl peptidase IV.[Pubmed:15556158]
Eur J Pharmacol. 2004 Nov 28;505(1-3):237-41.
K579 ((S)-1-[4-methyl-1-(2-pyrimidinyl)-4-piperidylamino]acetyl-2-pyrrolidinecarbonitr ile), which is a long-acting and a slow binding dipeptidyl peptidase IV inhibitor, preserved the endogenously secreted active forms of glucagon-like peptide-1, augmented the insulin response and ameliorated the glucose excursion during oral glucose tolerance test in rats. In this study, we measured plasma concentrations of K579 after oral administration to rats. However, K579 was eliminated rapidly from plasma after oral administration to rats. Therefore, we postulated that there are active metabolites of K579 in rat plasma. We investigated the effect of K579 on plasma dipeptidyl peptidase IV activity using bile duct-cannulated rats. The duration of inhibitory action of plasma dipeptidyl peptidase IV after the administration of K579 in bile duct-cannulated rats was shorter than that in sham-operated rats. Moreover, we investigated the effect of bile obtained from K579-treated rat on plasma dipeptidyl peptidase IV activity in normal rats. The bile collected from K579-treated rats exhibited tardive and potent inhibitory activity of normal rat plasma. These results suggest that K579 sustained the duration of inhibitory action of plasma dipeptidyl peptidase IV by the character as a slow-binding inhibitor and, as well, by the presence of metabolites of K579, which exhibit the inhibitory activity of dipeptidyl peptidase IV.


