IvangustinCAS# 14164-59-1 |
Quality Control & MSDS
Number of papers citing our products

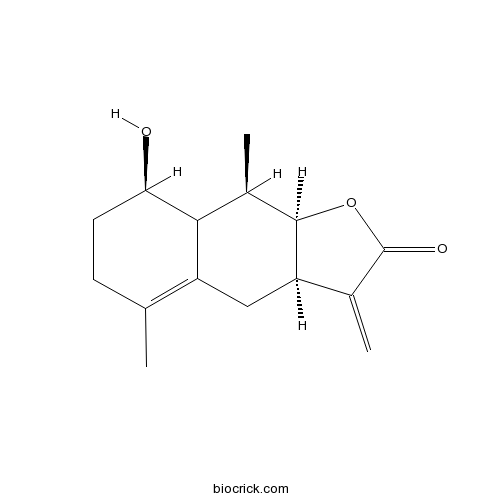
Chemical structure

3D structure
| Cas No. | 14164-59-1 | SDF | Download SDF |
| PubChem ID | 102004713 | Appearance | Powder |
| Formula | C15H20O3 | M.Wt | 248.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3aR,8R,9R,9aR)-8-hydroxy-5,9-dimethyl-3-methylidene-3a,4,6,7,8,8a,9,9a-octahydrobenzo[f][1]benzofuran-2-one | ||
| SMILES | CC1C2C(CCC(=C2CC3C1OC(=O)C3=C)C)O | ||
| Standard InChIKey | CHKRTCRFGDZZPE-JVBDDDBESA-N | ||
| Standard InChI | InChI=1S/C15H20O3/c1-7-4-5-12(16)13-9(3)14-11(6-10(7)13)8(2)15(17)18-14/h9,11-14,16H,2,4-6H2,1,3H3/t9-,11-,12-,13?,14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Ivangustin exhibits remarkable cytotoxicity against HEp2, SGC-7901 and HCT116 human cancer cell lines. |
| Targets | PARP |

Ivangustin Dilution Calculator

Ivangustin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0274 mL | 20.1369 mL | 40.2739 mL | 80.5477 mL | 100.6847 mL |
| 5 mM | 0.8055 mL | 4.0274 mL | 8.0548 mL | 16.1095 mL | 20.1369 mL |
| 10 mM | 0.4027 mL | 2.0137 mL | 4.0274 mL | 8.0548 mL | 10.0685 mL |
| 50 mM | 0.0805 mL | 0.4027 mL | 0.8055 mL | 1.611 mL | 2.0137 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4027 mL | 0.8055 mL | 1.0068 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GR 94800
Catalog No.:BCC5799
CAS No.:141636-65-9
- CU CPT 22
Catalog No.:BCC6320
CAS No.:1416324-85-0
- Dronedarone
Catalog No.:BCN2176
CAS No.:141626-36-0
- (±)-BI-D
Catalog No.:BCC5537
CAS No.:1416258-16-6
- Dronedarone HCl
Catalog No.:BCC4777
CAS No.:141625-93-6
- Beta-D-glucopyranosyl oleanolate
Catalog No.:BCN6530
CAS No.:14162-53-9
- JW 642
Catalog No.:BCC6324
CAS No.:1416133-89-5
- UNC1215
Catalog No.:BCC2023
CAS No.:1415800-43-9
- Angustin B
Catalog No.:BCN7652
CAS No.:1415795-51-5
- Angustin A
Catalog No.:BCN7651
CAS No.:1415795-50-4
- ST-836 hydrochloride
Catalog No.:BCC1969
CAS No.:1415564-68-9
- PF-543 Citrate
Catalog No.:BCC1855
CAS No.:1415562-83-2
- Thrombin Receptor Activator for Peptide 5 (TRAP-5)
Catalog No.:BCC1025
CAS No.:141685-53-2
- NPEC-caged-D-AP5
Catalog No.:BCC7895
CAS No.:1416943-27-5
- Galanin (2-29) (rat)
Catalog No.:BCC5763
CAS No.:141696-11-9
- Faropenem daloxate
Catalog No.:BCC1571
CAS No.:141702-36-5
- RI-2
Catalog No.:BCC6424
CAS No.:1417162-36-7
- H-Phe(4-Cl)-OH
Catalog No.:BCC3171
CAS No.:14173-39-8
- H-DL-Phe(4-Cl)-OMe.HCl
Catalog No.:BCC3175
CAS No.:14173-40-1
- Exenatide acetate
Catalog No.:BCN8514
CAS No.:141732-76-5
- MM-102
Catalog No.:BCC4551
CAS No.:1417329-24-8
- SDZ SER 082 fumarate
Catalog No.:BCC6994
CAS No.:1417343-80-6
- Exendin-4
Catalog No.:BCC1568
CAS No.:141758-74-9
- Albatrelin A
Catalog No.:BCN7626
CAS No.:1417805-15-2
Cytotoxic and Pro-apoptotic Activities of Sesquiterpene Lactones from Inula britannica.[Pubmed:26996005]
Nat Prod Commun. 2016 Jan;11(1):7-10.
In this study, five known sesquiterpene lactones (STL) with an alpha-methylene-gamma-lactone motif, including two eudesmanolides, 1beta-hydroxyalantolactone (1) and Ivangustin (2), and three 1,10-seco-eudesmanolides, 1-O-acetylbritannilactone (3), 1,6-O,O-diacetylbritannilactone (4), and 6alpha-O-(2- methylbutyryl)britannilactone (5) were isolated from the flower heads of the medicinal plant Inula britannica. Their structures were characterized by spectroscopic methods. X-ray data of 2 is reported for the first time. Among them, eudesmanolides 1 and 2 exhibited remarkable cytotoxicity against HEp2, SGC-7901 and HCT116 human cancer cell lines, comparable with etoposide (Vp-16) used as reference drug. Furthermore, treatment of HEp2 cells with 1 induced apoptosis associated with cleaved procaspase-3 and PARP. The biological assays carried out with normal cells (CHO) revealed that all sesquiterpenes were weakly selective against the cancer cell lines tested.
Simultaneous determination of three sesquiterpene lactones from Herba Inula extract in rat plasma by LC/MS/MS and its application to pharmacokinetic study.[Pubmed:22819204]
J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Aug 15;903:40-5.
A rapid and sensitive liquid chromatography-tandem mass spectrometry method has been developed and validated for the determination of 1-acetoxy-6alpha-hydroxyeriolanolide, 1beta-hydroxyalantolactone and Ivangustin from Herba Inula extract in rat plasma. Plasma samples were pretreated by protein precipitation with methanol. Chromatographic separation was accomplished on a TOSOH TSKgel ODS column with mobile phase consisting of methanol and 0.3% formic acid (80:20, v/v). The detection was carried out by multiple-reaction monitoring mode under positive electrospray ionization. The quantification was performed using the transitions of m/z 309.1/185.0 for 1-acetoxy-6alpha-hydroxyeriolanolide, m/z 249.0/231.1 for 1beta-hydroxyalantolactone and Ivangustin and m/z 285.0/193.0 for diazepam, respectively. Calibration curves were linear over the concentration range of 4-800 ng/mL for 1-acetoxy-6alpha-hydroxyeriolanolide, 8-500 ng/mL for 1beta-hydroxyalantolactone and Ivangustin. The limit of detection (LOD) was 1 ng/mL for 1-acetoxy-6alpha-hydroxyeriolanolide, 1.6 ng/mL for 1beta-hydroxyalantolactone and Ivangustin (S/N=3). The intra-day and inter-day precisions (RSD%) for the three compounds were less than 7.8% and 8.6%, and the accuracy (RE%) ranged from -4.6 to 6.8%. The method was successfully applied to pharmacokinetic studies of the three sesquiterpene lactones after oral administration of 300 mg/kg Herba Inula extract to rats, the t((1/2)) of 1-acetoxy-6alpha-hydroxyeriolanolide, 1beta-hydroxyalantolactone and Ivangustin was 9.65+/-1.43, 14.88+/-0.82 and 13.93+/-2.74 (h). The AUC((0-t)) of 1-acetoxy-6alpha-hydroxyeriolanolide, 1beta-hydroxyalantolactone and Ivangustin was 1102.46+/-247.04, 808.92+/-117.53 and 990.35+/-275.49 (ng h/mL), respectively.
Synthesis, cytotoxicity and inhibition of NO production of ivangustin enantiomer analogues.[Pubmed:26280922]
Eur J Med Chem. 2015 Sep 18;102:256-65.
The eight novel Ivangustin enantiomer analogues possessing alpha-methylene-gamma-butyrolactone moiety have been synthesized using (4S6R, 4S6S)-4-tert-butyldimethylsilyloxy-6-methylcyclohex-2-en-1-one (1) as starting material. These transformations were mainly carried out by aldol condensation reaction and one-pot annelation procedure. The stereochemistry of these synthesized analogues was determined by NOE analysis. Their cytoxicity was evaluated against the human cancer cell lines HCT-116 (colon), HL-60 (leukemia), QGY-7701 (liver), SMMC-7721 (liver), A549 (lung), MCF-7 (breast). The results showed that these analogues were more selective against the cell lines HL-60 and QGY-7701. Analogue 17 exhibited potent cytotoxicity and high selectivity toward HL-60 cell line with IC50 value of 1.02 muM, which suggested that it might be a promising anti-cancer lead compound. The inhibitory activities against NO production and the cytotoxicities in RAW 264.7 macrophages were determined at the same time. All of the analogues significantly inhibited the NO production with IC50 value in the range of 3.44-6.99 muM. Analogues 17, 22, 23 and 7 showed higher cytotoxicities, indicated their inhibitory activities against NO production may be influenced by the cytotoxicities.


