Glochicoccin DCAS# 927812-23-5 |
Quality Control & MSDS
Number of papers citing our products

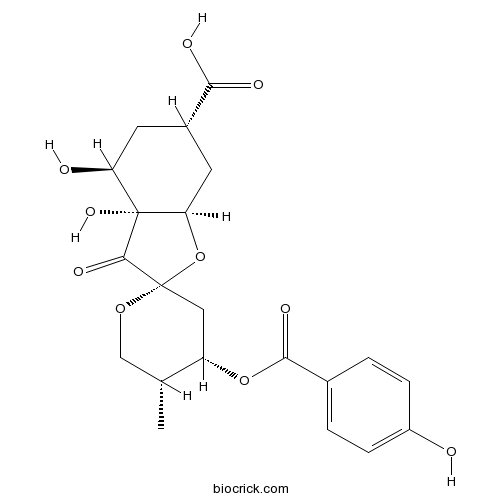
Chemical structure

3D structure
| Cas No. | 927812-23-5 | SDF | Download SDF |
| PubChem ID | 91895381 | Appearance | Powder |
| Formula | C21H24O10 | M.Wt | 436.4 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3aR,4S,4'S,5'R,6S,7aR)-3a,4-dihydroxy-4'-(4-hydroxybenzoyl)oxy-5'-methyl-3-oxospiro[5,6,7,7a-tetrahydro-4H-1-benzofuran-2,2'-oxane]-6-carboxylic acid | ||
| SMILES | CC1COC2(CC1OC(=O)C3=CC=C(C=C3)O)C(=O)C4(C(CC(CC4O2)C(=O)O)O)O | ||
| Standard InChIKey | ABMLTSFSLUQUFY-XUAJAPSHSA-N | ||
| Standard InChI | InChI=1S/C21H24O10/c1-10-9-29-20(8-14(10)30-18(26)11-2-4-13(22)5-3-11)19(27)21(28)15(23)6-12(17(24)25)7-16(21)31-20/h2-5,10,12,14-16,22-23,28H,6-9H2,1H3,(H,24,25)/t10-,12+,14+,15+,16-,20+,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| In vitro | Anti-hepatitis B virus activities and absolute configurations of sesquiterpenoid glycosides from Phyllanthus emblica.[Pubmed: 25268491]Org Biomol Chem. 2014 Nov 21;12(43):8764-74.
|
| Structure Identification | J Org Chem. 2010 Nov 5;75(21):7461-4.Stereoselective α,α'-annelation reactions of 1,3-dioxan-5-ones.[Pubmed: 20936869 ]
|

Glochicoccin D Dilution Calculator

Glochicoccin D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2915 mL | 11.4574 mL | 22.9148 mL | 45.8295 mL | 57.2869 mL |
| 5 mM | 0.4583 mL | 2.2915 mL | 4.583 mL | 9.1659 mL | 11.4574 mL |
| 10 mM | 0.2291 mL | 1.1457 mL | 2.2915 mL | 4.583 mL | 5.7287 mL |
| 50 mM | 0.0458 mL | 0.2291 mL | 0.4583 mL | 0.9166 mL | 1.1457 mL |
| 100 mM | 0.0229 mL | 0.1146 mL | 0.2291 mL | 0.4583 mL | 0.5729 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Motolimod (VTX-2337)
Catalog No.:BCC6497
CAS No.:926927-61-9
- Toll-like receptor modulator
Catalog No.:BCC2007
CAS No.:926927-42-6
- Germacrone 4,5-epoxide
Catalog No.:BCN6724
CAS No.:92691-35-5
- Deltatsine
Catalog No.:BCN8106
CAS No.:92631-66-8
- GGsTop
Catalog No.:BCC6187
CAS No.:926281-37-0
- BG45
Catalog No.:BCC6469
CAS No.:926259-99-6
- Milnacipran
Catalog No.:BCC4194
CAS No.:92623-85-3
- O-Demethylforbexanthone
Catalog No.:BCN4469
CAS No.:92609-77-3
- Radotinib(IY-5511)
Catalog No.:BCC6398
CAS No.:926037-48-1
- AMTB hydrochloride
Catalog No.:BCC7834
CAS No.:926023-82-7
- Dayecrystal A
Catalog No.:BCN4859
CAS No.:926010-24-4
- Secaubrytriol
Catalog No.:BCN4468
CAS No.:925932-10-1
- KC7F2
Catalog No.:BCC2434
CAS No.:927822-86-4
- DPNI-caged-GABA
Catalog No.:BCC5957
CAS No.:927866-58-8
- RAF265
Catalog No.:BCC3677
CAS No.:927880-90-8
- Golvatinib (E7050)
Catalog No.:BCC4423
CAS No.:928037-13-2
- Alisol O
Catalog No.:BCN3362
CAS No.:928148-51-0
- Tenacissoside F
Catalog No.:BCN4472
CAS No.:928151-78-4
- 3-Chloro-1-(4-octylphenyl)-propanone
Catalog No.:BCN2249
CAS No.:928165-59-7
- Boc-D-Asp-OBzl
Catalog No.:BCC3370
CAS No.:92828-64-3
- MN 64
Catalog No.:BCC6489
CAS No.:92831-11-3
- AS 1892802
Catalog No.:BCC6335
CAS No.:928320-12-1
- IRAK inhibitor 2
Catalog No.:BCC1655
CAS No.:928333-30-6
- PF-03716556
Catalog No.:BCC2084
CAS No.:928774-43-0
Anti-hepatitis B virus activities and absolute configurations of sesquiterpenoid glycosides from Phyllanthus emblica.[Pubmed:25268491]
Org Biomol Chem. 2014 Nov 21;12(43):8764-74.
During the process exploring anti-viral compounds from Phyllanthus species, eight new highly oxygenated bisabolane sesquiterpenoid glycoside phyllaemblicins G1-G8 (1-8) were isolated from Phyllanthus emblica, along with three known compounds, phyllaemblicin F (9), phyllaemblic acid (10) and Glochicoccin D (11). Phyllaemblicin G2 (2), bearing a tricyclo [3.1.1.1] oxygen bridge ring system, is an unusual sesquiterpenoid glycoside, while phyllaemblicins G6-G8 (6-8) are dimeric sesquiterpenoid glycosides with two norbisabolane units connecting through a disaccharide. All the structures were elucidated by the extensive analysis of HRMS and NMR data. The relative configuration of phyllaemblicin G2 was constructed based on heteronuclear coupling constants measurement, and the absolute configurations for all new compounds were established by calculated electronic circular dichroism (ECD) using time dependent density functional theory. The sesquiterpenoid glycoside dimers 6-9 displayed potential anti-hepatitis B virus (HBV) activities, especially for the new compound 6 with IC50 of 8.53 +/- 0.97 and 5.68 +/- 1.75 muM towards the HBV surface antigen (HBsAg) and HBV excreted antigen (HBeAg) secretion, respectively.


