Dihydroconiferyl alcoholCAS# 2305-13-7 |
Quality Control & MSDS
Number of papers citing our products

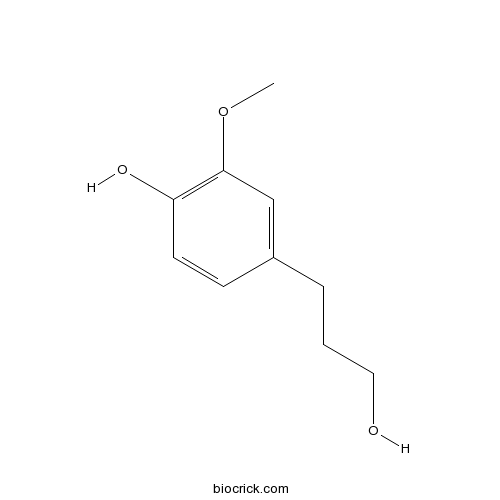
Chemical structure

3D structure
| Cas No. | 2305-13-7 | SDF | Download SDF |
| PubChem ID | 16822 | Appearance | Oil |
| Formula | C10H14O3 | M.Wt | 182.21 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-(3-hydroxypropyl)-2-methoxyphenol | ||
| SMILES | COC1=C(C=CC(=C1)CCCO)O | ||
| Standard InChIKey | MWOMNLDJNQWJMK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H14O3/c1-13-10-7-8(3-2-6-11)4-5-9(10)12/h4-5,7,11-12H,2-3,6H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Dihydroconiferyl alcohol exhibits cytoprotective activity in cultured MCF-7 cells stressed by H2O2. |

Dihydroconiferyl alcohol Dilution Calculator

Dihydroconiferyl alcohol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4882 mL | 27.4409 mL | 54.8817 mL | 109.7635 mL | 137.2043 mL |
| 5 mM | 1.0976 mL | 5.4882 mL | 10.9763 mL | 21.9527 mL | 27.4409 mL |
| 10 mM | 0.5488 mL | 2.7441 mL | 5.4882 mL | 10.9763 mL | 13.7204 mL |
| 50 mM | 0.1098 mL | 0.5488 mL | 1.0976 mL | 2.1953 mL | 2.7441 mL |
| 100 mM | 0.0549 mL | 0.2744 mL | 0.5488 mL | 1.0976 mL | 1.372 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lofepramine
Catalog No.:BCC7402
CAS No.:23047-25-8
- Ajugasterone C
Catalog No.:BCN2757
CAS No.:23044-80-6
- Z-Arg(NO2)-OH
Catalog No.:BCC3063
CAS No.:2304-98-5
- Z-Asn-OH
Catalog No.:BCC2794
CAS No.:2304-96-3
- Z-β-Ala-OH
Catalog No.:BCC3058
CAS No.:2304-94-1
- Terbutaline Sulfate
Catalog No.:BCC4320
CAS No.:23031-32-5
- Apelin-36 (rat, mouse)
Catalog No.:BCC5911
CAS No.:230299-95-3
- Physalin A
Catalog No.:BCN7920
CAS No.:23027-91-0
- Neoglycyrol
Catalog No.:BCN2907
CAS No.:23013-84-5
- MPTP hydrochloride
Catalog No.:BCC1778
CAS No.:23007-85-4
- Atazanavir sulfate (BMS-232632-05)
Catalog No.:BCC2114
CAS No.:229975-97-7
- AGN 194310
Catalog No.:BCC5416
CAS No.:229961-45-9
- L-AP4
Catalog No.:BCC6550
CAS No.:23052-81-5
- (-)-Sophoranone
Catalog No.:BCN7162
CAS No.:23057-55-8
- Sinensetin
Catalog No.:BCN6356
CAS No.:2306-27-6
- Varenicline Hydrochloride
Catalog No.:BCC4156
CAS No.:230615-23-3
- Eurycomalactone
Catalog No.:BCN3108
CAS No.:23062-24-0
- PD 102807
Catalog No.:BCC7145
CAS No.:23062-91-1
- 4-Amino-N-methylphthalimide
Catalog No.:BCC8686
CAS No.:2307-00-8
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Sitosteryl palmitate
Catalog No.:BCN5078
CAS No.:2308-85-2
- 2-amino-3-(3-bromo-5-chloro-4-hydroxyphenyl)propanoic acid
Catalog No.:BCN8284
CAS No.:
- Corilagin
Catalog No.:BCN2322
CAS No.:23094-69-1
- Chebulagic acid
Catalog No.:BCN3262
CAS No.:23094-71-5
Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds.[Pubmed:21033720]
J Agric Food Chem. 2010 Nov 24;58(22):11673-9.
Twenty-three phenolic compounds were isolated from a butanol extract of Canadian maple syrup (MS-BuOH) using chromatographic methods. The compounds were identified from their nuclear magnetic resonance and mass spectral data as 7 lignans [lyoniresinol (1), secoisolariciresinol (2), dehydroconiferyl alcohol (3), 5'-methoxy-dehydroconiferyl alcohol (4), erythro-guaiacylglycerol-beta-O-4'-coniferyl alcohol (5), erythro-guaiacylglycerol-beta-O-4'-Dihydroconiferyl alcohol (6), and [3-[4-[(6-deoxy-alpha-l-mannopyranosyl)oxy]-3-methoxyphenyl]methyl]-5-(3,4-dimeth oxyphenyl)dihydro-3-hydroxy-4-(hydroxymethyl)-2(3H)-furanone (7)], 2 coumarins [scopoletin (8) and fraxetin (9)], a stilbene [(E)-3,3'-dimethoxy-4,4'-dihydroxystilbene (10)], and 13 phenolic derivatives [2-hydroxy-3',4'-dihydroxyacetophenone (11), 1-(2,3,4-trihydroxy-5-methylphenyl)ethanone (12), 2,4,5-trihydroxyacetophenone (13), catechaldehyde (14), vanillin (15), syringaldehyde (16), gallic acid (17), trimethyl gallic acid methyl ester (18), syringic acid (19), syringenin (20), (E)-coniferol (21), C-veratroylglycol (22), and catechol (23)]. The antioxidant activities of MS-BuOH (IC50>1000 mug/mL), pure compounds, vitamin C (IC50=58 muM), and a synthetic commercial antioxidant, butylated hydroxytoluene (IC50=2651 muM), were evaluated in the diphenylpicrylhydrazyl (DPPH) radical scavenging assay. Among the isolates, the phenolic derivatives and coumarins showed superior antioxidant activity (IC50<100 muM) compared to the lignans and stilbene (IC50>100 muM). Also, this is the first report of 16 of these 23 phenolics, that is, compounds 1, 2, 4-14, 18, 20, and 22, in maple syrup.
Inositol Derivatives and Phenolic Compounds from the Roots of Taraxacum coreanum.[Pubmed:28805750]
Molecules. 2017 Aug 14;22(8). pii: molecules22081349.
In this study, the characterization of chemical constituents and biological activity of the roots of Taraxacum coreanum (Asteraceae) was attempted. Phytochemical investigation of the roots of T. coreanum led to the isolation of two new inositol derivatives, taraxinositols A (1) and B (2), and a new phenolic compound, taraxinol (16), together with twenty known compounds including four inositol derivatives, neo-inositol-1,4-bis (4-hydroxybenzeneacetate) (3), chiro-inositol-1,5-bis(4- hydroxybenzeneacetate) (4), chiro-inositol-2,3-bis (4-hydroxybenzeneacetate) (5) and chiro-inositol- 1,2,3-tris (4-hydroxybenzeneacetate) (6), nine phenolic compounds: p-hydroxybenzaldehyde (7), vanillin (8), syringaldehyde (9), vanillic acid (10), 4-methoxyphenylacetic acid (11), 4-hydroxy- phenylacetic acid methyl ester (12), optivanin (13), isoferulic acid (14) and Dihydroconiferyl alcohol (15), four coumarins: nodakenetin (17), decursinol (18), prangol (19) and isobyakangelicin (20), and three lignans: syringaresinol-4'-O-beta-d-glucoside (21), syringaresinol (22), and pinoresinol (23). The structures of isolated compounds were determined on the basis of spectroscopic analysis. Among the isolated compounds, vanillic acid, isoferulic acid and syringaresinol showed radical scavenging activity with IC50 values ranging from 30.4 to 75.2 muM.
[Chemical consitituents from root of Isatis indigotica].[Pubmed:23944031]
Zhongguo Zhong Yao Za Zhi. 2013 Apr;38(8):1172-82.
Thirty-three compounds were isolated from the root decoction of Isatis indigotica by using a combination of various chromatographic techniques including silica gel, macroporous adsorbent resin, Sephadex LH-20, and reversed-phase HPLC. Their structures were elucidated by spectroscopic data as (+)-dehydrovomifoliol (1), (S)-(+)-abscisic acid (2), vomifoliol (3), cyclo (L-Phe-L-Leu) (4), cyclo(L-Phe-L-Tyr) (5), cyclo(L-Tyr-L-Leu) (6), cyclo(L-Pro-L-Tyr) (7), evofolin B (8), (+)-syringaresinol (9), (-)-(7R,7'R,8S,8'S)-4,4'-dihydroxy-3-methoxy-7,9';7',9-diepoxy-lignan (10), (-)-medioresinol (11), (+) -(7R,7'R,8S,8'S) -neo-olivil (12), (-) -5-methoxyisolariciresinol (13), 1,3-dihydro-2H-indol-2-one (14), isalexin (15), dihydroneoascorbigen (16), indican (17), (-) -(S) -cyanomethyl-3-hydroxyoxindole (18), isoformononetein (19), calycosin (20), stigamast-5-ene-3beta-ol-7-one (21), acetovanillone (22), 3, 5-dimethoxy-4-hydroxyacetophenone (23), Dihydroconiferyl alcohol (24), dihyroferulic acid (25), 3-hydroxy-1-(4-hydroxyphenyl) propan-1-one (26), beta-hydroxypropiovanillone (27), 4-aminobenzoic acid (28), 3-(4-hydroxyphenyl) propan-1-ol (29), 4-(2-hydroxyethyl) phenol (30), 2-methoxy-4-vinylphenol (31), pyrocatechol (32), and 4-pentenamide (33). These compounds were isolated from the root of I. indigotica for the first time. In preliminary in vitro assays, compound 19 showed activity against the influenza virus A/Hanfang/359/95 (H3N2), the herpes simplex virus 1 (HSV-1), and Coxsackie virus B3 (Cox-B3), with IC50 values of 2.06, 6.84, and 8.70 micromol x L(-1), respectively, but other compounds were in-active at a concentration of 1.0 x 10 x (-5) mol x L(-1).
Further investigation into maple syrup yields 3 new lignans, a new phenylpropanoid, and 26 other phytochemicals.[Pubmed:21675726]
J Agric Food Chem. 2011 Jul 27;59(14):7708-16.
Maple syrup is made by boiling the sap collected from certain maple ( Acer ) species. During this process, phytochemicals naturally present in tree sap are concentrated in maple syrup. Twenty-three phytochemicals from a butanol extract of Canadian maple syrup (MS-BuOH) had previously been reported; this paper reports the isolation and identification of 30 additional compounds (1-30) from its ethyl acetate extract (MS-EtOAc) not previously reported from MS-BuOH. Of these, 4 compounds are new (1-3, 18) and 20 compounds (4-7, 10-12, 14-17, 19, 20, 22-24, 26, and 28-30) are being reported from maple syrup for the first time. The new compounds include 3 lignans and 1 phenylpropanoid: 5-(3'',4''-dimethoxyphenyl)-3-hydroxy-3-(4'-hydroxy-3'-methoxybenzyl)-4-(hydroxym ethyl)dihydrofuran-2-one (1), (erythro,erythro)-1-[4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl) ethoxy]-3,5-dimethoxyphenyl]-1,2,3-propanetriol (2), (erythro,threo)-1-[4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)et hoxy]-3,5-dimethoxyphenyl]-1,2,3-propanetriol (3), and 2,3-dihydroxy-1-(3,4- dihydroxyphenyl)-1-propanone (18), respectively. In addition, 25 other phenolic compounds were isolated including (threo,erythro)-1-[4-[(2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)e thoxy]-3-methoxyphenyl]-1,2,3-propanetriol (4), (threo,threo)-1-[4-[(2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)eth oxy]-3-methoxyphenyl]-1,2,3-propanetriol (5), threo-guaiacylglycerol-beta-O-4'-Dihydroconiferyl alcohol (6), erythro-1-(4-hydroxy-3-methoxyphenyl)-2-[4-(3-hydroxypropyl)-2,6-dimethoxyphenoxy ]-1,3-propanediol (7), 2-[4-[2,3-dihydro-3-(hydroxymethyl)-5-(3-hydroxypropyl)-7-methoxy-2-benzofuranyl] -2,6-dimethoxyphenoxy]-1-(4-hydroxy-3-methoxyphenyl)-1,3-propanediol (8), acernikol (9), leptolepisol D (10), buddlenol E (11), (1S,2R)-2-[2,6-dimethoxy-4-[(1S,3aR,4S,6aR)-tetrahydro-4-(4-hydroxy-3,5-dimethoxy phenyl)-1H,3H-furo[3,4-c]furan-1-yl]phenoxy]-1-(4-hydroxy-3-methoxyphenyl)-1,3-pr opanediol (12), syringaresinol (13), isolariciresinol (14), icariside E4 (15), sakuraresinol (16), 1,2-diguaiacyl-1,3-propanediol (17), 2,3-dihydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-1-propanone (19), 3-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)propan-1-one (20), Dihydroconiferyl alcohol (21), 4-acetylcatechol (22), 3',4',5'-trihydroxyacetophenone (23), 3,4-dihydroxy-2-methylbenzaldehyde (24), protocatechuic acid (25), 4-(dimethoxymethyl)pyrocatechol (26), tyrosol (27), isofraxidin (28), and 4-hydroxycatechol (29). One sesquiterpene, phaseic acid (30), which is a known metabolite of the phytohormone abscisic acid, was also isolated from MS-EtOAc. The antioxidant activities of MS-EtOAc (IC(50) = 75.5 mug/mL) and the pure isolates (IC(50) ca. 68-3000 muM) were comparable to that of vitamin C (IC(50) = 40 muM) and the synthetic commercial antioxidant butylated hydroxytoluene (IC(50) = 3000 muM), in the diphenylpicrylhydrazyl radical scavenging assay. The current study advances scientific knowledge of maple syrup constituents and suggests that these diverse phytochemicals may impart potential health benefits to this natural sweetener.


