Cytochrome c - pigeon (88-104)Triggers T Cell response CAS# 86579-06-8 |
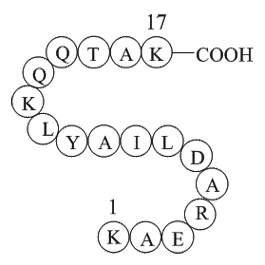
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure
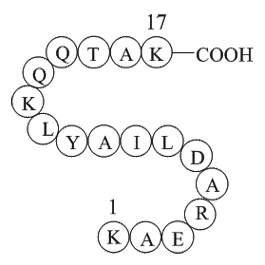
3D structure
| Cas No. | 86579-06-8 | SDF | Download SDF |
| PubChem ID | 71464382 | Appearance | Powder |
| Formula | C84H144N24O25 | M.Wt | 1890.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Sequence | H2N-Lys-Ala-Glu-Arg-Ala-Asp-Leu-Ile-Ala-Tyr-Leu-Lys-Gln-Ala-Thr-Ala-Lys-OH | ||
| SMILES | CCC(C)C(C(=O)NC(C)C(=O)NC(CC1=CC=C(C=C1)O)C(=O)NC(CC(C)C)C(=O)NC(CCCCN)C(=O)NC(CCC(=O)N)C(=O)NC(C)C(=O)NC(C(C)O)C(=O)NC(C)C(=O)NC(CCCCN)C(=O)O)NC(=O)C(CC(C)C)NC(=O)C(CC(=O)O)NC(=O)C(C)NC(=O)C(CCCNC(=N)N)NC(=O)C(CCC(=O)O)NC(=O)C(C)NC(=O)C(CCCCN)N | ||
| Standard InChIKey | UZAAFHHXLLVUAZ-XKHMURAPSA-N | ||
| Standard InChI | InChI=1S/C84H144N24O25/c1-13-43(6)65(107-80(129)59(38-42(4)5)106-79(128)61(40-64(114)115)104-69(118)45(8)94-73(122)54(24-20-36-92-84(90)91)99-76(125)56(30-32-63(112)113)98-67(116)44(7)93-72(121)52(88)21-14-17-33-85)81(130)96-47(10)70(119)103-60(39-50-25-27-51(110)28-26-50)78(127)105-58(37-41(2)3)77(126)100-53(22-15-18-34-86)75(124)101-55(29-31-62(89)111)74(123)95-48(11)71(120)108-66(49(12)109)82(131)97-46(9)68(117)102-57(83(132)133)23-16-19-35-87/h25-28,41-49,52-61,65-66,109-110H,13-24,29-40,85-88H2,1-12H3,(H2,89,111)(H,93,121)(H,94,122)(H,95,123)(H,96,130)(H,97,131)(H,98,116)(H,99,125)(H,100,126)(H,101,124)(H,102,117)(H,103,119)(H,104,118)(H,105,127)(H,106,128)(H,107,129)(H,108,120)(H,112,113)(H,114,115)(H,132,133)(H4,90,91,92)/t43-,44-,45-,46-,47-,48-,49+,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,65-,66-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cytochrome c - pigeon (88-104) Dilution Calculator

Cytochrome c - pigeon (88-104) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Lys-Ala-Glu-Arg-Ala-Asp-Leu-Ile-Ala-Tyr-Leu-Lys-Gln-Ala-Thr-Ala-Lys-OH
The I-Ek-restricted T cell response to Cytochrome c - pigeon (pcyt c) is specific for a peptide within the COOH-terminal sequence 88-1041.
The minimal length of the pcyt c peptide required for T cell stimulation is the COOH-terminal sequence containing residues 95-104. However, the addition of residues to the NH2 terminus of residue 95 increases the antigenic potency of the peptide; the maximal response is induced by pcyt c 88-1042.
The substitution of any residue between 95 and 104 of the cytochrome c peptide changed the antigenic potency of the peptide for at least one of the hybridomas3.
Figure1. Structure of Cytochrome c
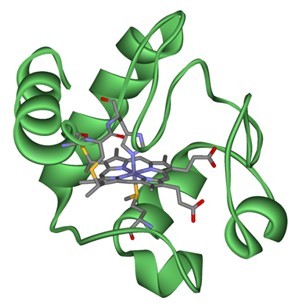
Figure2. Formula of Cytochrome c - pigeon (88-104)
C84H144N24O25
Ref:
1. Schwartz, R. H. 1985. T lymphocyte recognition of antigen in association with gene products of the MHC. Annu. Rev. Immunol. 3:237.
2. Bhayani, H., F. R. Carbone, and Y. Paterson. 1988. The activation of pigeon cytochrome c-specific T cell hybridomas by antigenic peptides is influenced by non-native sequences at the amino terminus of the determinant. J. Immunol. 141:377.
3. Bhayani, H., Y. Paterson. 1989. Analysis of peptide binding patterns in different major histocompatibility complex/t cell receptor complexes using pigeon cytochrome c-specific t cell hybridomas.
- PF-03084014
Catalog No.:BCC1848
CAS No.:865773-15-5
- Trelagliptin
Catalog No.:BCC2014
CAS No.:865759-25-7
- 2-[(6-Chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]benzonitrile
Catalog No.:BCC8506
CAS No.:865758-96-9
- TC-E 5001
Catalog No.:BCC6355
CAS No.:865565-29-3
- Ganoderic acid SZ
Catalog No.:BCN4413
CAS No.:865543-37-9
- Junipediol A
Catalog No.:BCN6912
CAS No.:86548-91-6
- 5-R-Rivaroxaban
Catalog No.:BCC1313
CAS No.:865479-71-6
- Bisisorhapontigenin A
Catalog No.:BCN3501
CAS No.:865474-98-2
- Narlaprevir
Catalog No.:BCC1785
CAS No.:865466-24-6
- apigenin 7-O-(6〃-O-malonyl)-β-D-glucoside
Catalog No.:BCN8399
CAS No.:86546-87-4
- Benazepril
Catalog No.:BCC4286
CAS No.:86541-75-5
- Benazepril HCl
Catalog No.:BCC5019
CAS No.:86541-74-4
- RX-3117
Catalog No.:BCC6381
CAS No.:865838-26-2
- Chamaejasmenin D
Catalog No.:BCN3046
CAS No.:865852-47-7
- Isochamaejasmenin B
Catalog No.:BCN3045
CAS No.:865852-48-8
- Tideglusib
Catalog No.:BCC4511
CAS No.:865854-05-3
- Oleuropeic acid 8-O-glucoside
Catalog No.:BCN4025
CAS No.:865887-46-3
- L-745,870 trihydrochloride
Catalog No.:BCC5695
CAS No.:866021-03-6
- 6-Benzoyl-5,7-dihydroxy-2,2-dimethylchromane
Catalog No.:BCN1326
CAS No.:86606-14-6
- Clausine Z
Catalog No.:BCN4414
CAS No.:866111-14-0
- PRX-08066
Catalog No.:BCC4209
CAS No.:866206-54-4
- PRX-08066 Maleic acid
Catalog No.:BCC1165
CAS No.:866206-55-5
- 3,4,5-Tricaffeoylquinic acid
Catalog No.:BCN2384
CAS No.:86632-03-3
- Xanthiside
Catalog No.:BCN2545
CAS No.:866366-86-1
Interplay between cell division and cell death during TCR triggering.[Pubmed:15307175]
Eur J Immunol. 2004 Sep;34(9):2430-8.
Cell death is crucial to avoid excessive T cell expansion. During primary T cell expansion in response to pathogen or after vaccination, the amount of foreign Ag determines the degree of clonal amplification and death. Here, we studied the balance between cell proliferation and death, as well as susceptibility to cell death, during TCR triggering. After priming of CD4 T cells from AND-TCR (Vbeta3, Valpha11)-transgenic mice with a high dose of pigeon cytochrome c peptide 88-104, the cell expansion rate was significantly reduced by marked clonal elimination compared to lower Ag doses, whereas the number of cell divisions reached was similar at all Ag doses. TCR re-engagement on activated T cells induced cell death, irrespective of the dose of Ag encountered during primary stimulation. Surprisingly, commitment to apoptosis occurred as early as the first division on all dividing cells both in vitro and in vivo. This phenomenon was highly selective, as activated but non-dividing cells did not undergo cell death, whereas cells that had divided once became susceptible to cell death. These findings have direct implications for the peripheral homeostatic mechanism following Ag challenge and for designing primary/booster vaccine strategies.
Naive CD4+ T cells from lupus-prone Fas-intact MRL mice display TCR-mediated hyperproliferation due to intrinsic threshold defects in activation.[Pubmed:15814741]
J Immunol. 2005 Apr 15;174(8):5100-9.
Autoreactive T cell activation is a consistent feature of murine lupus; however, the mechanism of such activation remains unclear. We hypothesized that naive CD4+ T cells in lupus have a lower threshold of activation through their TCR-CD3 complex that renders them more susceptible to stimulation with self-Ags. To test this hypothesis, we compared proliferation, IL-2 production, and single cell calcium signaling of naive CD4+ T cells isolated from Fas-intact MRL/+(Fas-lpr) mice with H-2k-matched B10.BR and CBA/CaJ controls, following anti-CD3 stimulation in the presence or absence of anti-CD28. We also assessed the responsiveness of naive CD4+ T cells isolated from Fas-intact MRL and control mice bearing a rearranged TCR specific for amino acids 88-104 of pigeon cytochrome c to cognate and low affinity peptide Ags presented by bone marrow-matured dendritic cells. TCR transgenic and wild-type CD4+ T cells from MRL mice displayed a lower threshold of activation than control cells, a response that was class II MHC dependent. The rise in intracellular calcium in MRL vs controls was enhanced and prolonged following anti-CD3 triggering, suggestive of proximal defects in TCR-engendered signaling as the mechanism for the observed hyperactivity. These findings were observed as early as 1-2 mo postweaning and, based on analysis of F1 T cells, appeared to be dominantly expressed. This genetically altered threshold for activation of MRL T cells, a consequence of a proximal defect in CD3-mediated signal transduction, may contribute to the abrogation of T cell tolerance to self-Ags in lupus.
Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway.[Pubmed:21078910]
J Immunol. 2010 Dec 15;185(12):7474-9.
Chronic inflammation can associate with autoreactive immune responses, including CD4(+) T cell responses to self-Ags. In this paper, we show that the adipocyte-derived proinflammatory hormone leptin can affect the survival and proliferation of autoreactive CD4(+) T cells in experimental autoimmune encephalomyelitis, an animal model of human multiple sclerosis. We found that myelin olygodendrocyte glycoprotein peptide 35-55 (MOG(35-55))-specific CD4(+) T cells from C57BL/6J wild-type mice could not transfer experimental autoimmune encephalomyelitis into leptin-deficient ob/ob mice. Such a finding was associated with a reduced proliferation of the transferred MOG(35-55)-reactive CD4(+) T cells, which had a reduced degradation of the cyclin-dependent kinase inhibitor p27(kip1) and ERK1/2 phosphorylation. The transferred cells displayed reduced Th1/Th17 responses and reduced delayed-type hypersensitivity. Moreover, MOG(35-55)-reactive CD4(+) T cells in ob/ob mice underwent apoptosis that associated with a downmodulation of Bcl-2. Similar results were observed in transgenic AND-TCR- mice carrying a TCR specific for the pigeon cytochrome c 88-104 peptide. These molecular events reveal a reduced activity of the nutrient/energy-sensing AKT/mammalian target of rapamycin pathway, which can be restored in vivo by exogenous leptin replacement. These results may help to explain a link between chronic inflammation and autoimmune T cell reactivity.
Altered immunogenicity of isoaspartate containing proteins.[Pubmed:17453712]
Autoimmunity. 2007 Mar;40(2):131-7.
The development of immune tolerance is dependent on the expression of self-peptides in the thymus and bone marrow during lymphocyte development. However, not all self-antigens are expressed in the thymus, particularly for proteins that become post-translationally modified during other biological processes in a cell. We have found that one such post-translational modification, the spontaneous conversion of an aspartic acid to isoaspartic acid (isoAsp), causes ignored self-antigens to become immunogenic. In order to determine the mechanism for this autoimmune response, pigeon cytochrome c peptide 88-104 (PCC p88-104) was synthesized with and without an isoaspartyl residue. Each form was digested with cathepsin D, an enzyme involved in antigen processing. The products of cathepsin digestion were dramatically different between the two forms of self-protein suggesting that cryptic self-peptides may be revealed to the immune system by natural modifications to self-proteins. This observation also held true if whole PCC protein contained isoaspartyl residues was digested with cathespsin D. Additionally, AND transgenic TCR T cells (recognizing PCC 88-104) proliferated to a greater extent in response to isoaspartyl PCC as compared to the normal form of PCC. These finding demonstrate the importance of post-translational modifications in shaping autoimmune responses in and the development of tolerance to self-proteins.
CD4+ T cells from lupus-prone mice avoid antigen-specific tolerance induction in vivo.[Pubmed:12517936]
J Immunol. 2003 Jan 15;170(2):741-8.
Activated T cells in spontaneous lupus presumably bypass normal tolerance mechanisms in the periphery, since thymic tolerance appears intact. To determine whether such T cells indeed avoid in vivo peripheral tolerance mechanisms, we assessed their activation and recall responses after in vivo Ag stimulation in the absence of exogenously supplied costimulatory signals. Naive CD4(+) AND (transgenic mice bearing rearranged TCR specific for pigeon cytochrome c, peptides 88-104) TCR-transgenic T cells, specific for pigeon cytochrome c, from lupus-prone Fas-intact MRL/Mp+(Fas-lpr) and from H-2(k)-matched control CBA/CaJ and B10.BR mice (MRL.AND, CBA.AND, and B10.AND, respectively) were adoptively transferred into (MRL x CBA)F(1) or (MRL x B10)F(1) recipients transgenically expressing membrane-bound pigeon cytochrome c as a self-Ag. MRL.AND and control CBA.AND and B10.AND-transgenic T cells were activated and divided after transfer, indicating encounter with their cognate Ag; however, T cells from CBA.AND and B10.AND mice were impaired in their ability to proliferate and produce IL-2 after challenge with pigeon cytochrome c in ex vivo recall assays, a typical phenotype of anergized cells. By contrast, MRL.AND T cells proliferated more, and a significantly higher percentage of such cells produced IL-2, compared with control T cells. This observation that MRL T cells avoided anergy induction in vivo was confirmed in an in vitro system where the cells were stimulated with an anti-CD3 in the absence of a costimulatory signal. These experiments provide direct evidence that CD4(+) T cells from Fas-intact lupus-prone MRL mice are more resistant than nonautoimmune control cells to anergy induction. Anergy avoidance in the periphery might contribute to the characteristic finding in lupus of inappropriate T cell activation in response to ubiquitous self-Ags.


