CefquinomeCAS# 84957-30-2 |
Quality Control & MSDS
Number of papers citing our products

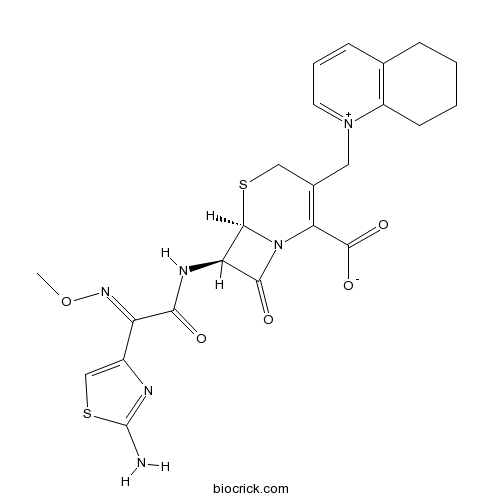
Chemical structure

3D structure
| Cas No. | 84957-30-2 | SDF | Download SDF |
| PubChem ID | 9571084 | Appearance | Powder |
| Formula | C23H24N6O5S2 | M.Wt | 528.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (6R,7R)-7-[[(2E)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-8-oxo-3-(5,6,7,8-tetrahydroquinolin-1-ium-1-ylmethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate | ||
| SMILES | CON=C(C1=CSC(=N1)N)C(=O)NC2C3N(C2=O)C(=C(CS3)C[N+]4=CC=CC5=C4CCCC5)C(=O)[O-] | ||
| Standard InChIKey | YWKJNRNSJKEFMK-KJXIDEHUSA-N | ||
| Standard InChI | InChI=1S/C23H24N6O5S2/c1-34-27-16(14-11-36-23(24)25-14)19(30)26-17-20(31)29-18(22(32)33)13(10-35-21(17)29)9-28-8-4-6-12-5-2-3-7-15(12)28/h4,6,8,11,17,21H,2-3,5,7,9-10H2,1H3,(H3-,24,25,26,30,32,33)/b27-16+/t17-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cefquinome Dilution Calculator

Cefquinome Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8918 mL | 9.4589 mL | 18.9179 mL | 37.8358 mL | 47.2947 mL |
| 5 mM | 0.3784 mL | 1.8918 mL | 3.7836 mL | 7.5672 mL | 9.4589 mL |
| 10 mM | 0.1892 mL | 0.9459 mL | 1.8918 mL | 3.7836 mL | 4.7295 mL |
| 50 mM | 0.0378 mL | 0.1892 mL | 0.3784 mL | 0.7567 mL | 0.9459 mL |
| 100 mM | 0.0189 mL | 0.0946 mL | 0.1892 mL | 0.3784 mL | 0.4729 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cevipabulin
Catalog No.:BCC6449
CAS No.:849550-05-6
- Cudraxanthone B
Catalog No.:BCN4398
CAS No.:84955-05-5
- Rosarin
Catalog No.:BCN5967
CAS No.:84954-93-8
- Rosavin
Catalog No.:BCN5968
CAS No.:84954-92-7
- TMPH hydrochloride
Catalog No.:BCC7360
CAS No.:849461-91-2
- 1-(4-Fluorobenzyl)-2-chlorobenzimidazole
Catalog No.:BCC8407
CAS No.:84946-20-3
- Grape Seed Extract
Catalog No.:BCC5317
CAS No.:84929-27-1
- Chinensine B
Catalog No.:BCN4397
CAS No.:849245-34-7
- Cabozantinib (XL184, BMS-907351)
Catalog No.:BCC1264
CAS No.:849217-68-1
- Foretinib (GSK1363089)
Catalog No.:BCC1263
CAS No.:849217-64-7
- Glaucogenin C mono-D-thevetoside
Catalog No.:BCN7089
CAS No.:849201-84-9
- Floribundasaponin A
Catalog No.:BCN1330
CAS No.:37341-36-9
- Blepharotriol
Catalog No.:BCN8056
CAS No.:849590-42-7
- SU14813 maleate
Catalog No.:BCC1973
CAS No.:849643-15-8
- Y 134
Catalog No.:BCC7451
CAS No.:849662-80-2
- Rubicordifolin
Catalog No.:BCN7154
CAS No.:849699-55-4
- Deapi-platycoside E
Catalog No.:BCN3320
CAS No.:849758-42-5
- ARP 101
Catalog No.:BCC2371
CAS No.:849773-63-3
- Maohuoside B
Catalog No.:BCN8084
CAS No.:849834-04-4
- 2-Benzoylbenzoic acid
Catalog No.:BCC8561
CAS No.:85-52-9
- Sudan IV;Solvent Red 24
Catalog No.:BCN8379
CAS No.:85-83-6
- Sudan III
Catalog No.:BCN6962
CAS No.:85-86-9
- Altrenogest
Catalog No.:BCC4479
CAS No.:850-52-2
- Methyl 1,6-dihydroxy-3-methylxanthone-8-carboxylate
Catalog No.:BCN7473
CAS No.:85003-85-6
Cefquinome-Loaded Microsphere Formulations in Protection against Pneumonia with Klebsiella pneumonia Infection and Inflammatory Response in Rats.[Pubmed:30923922]
Pharm Res. 2019 Mar 28;36(5):74.
PURPOSE: This study aimed to compare in vivo activity between Cefquinome (CEQ)-loaded poly lactic-co-glycolic acid (PLGA) microspheres (CEQ-PLGA-MS) and CEQ injection (CEQ-INJ) against Klebsiella pneumonia in a rat lung infection model. METHODS: Forty-eight rats were divided into control group (sham operated without infection and drug treatment), Klebsiella pneumonia model group (KPD + Saline), CEQ-PLGA-MS and CEQ-INJ therapy groups (KPD + CEQ-PLGA-MS and KPD + INJ, respectively). In the KPD + Saline group, rats were infected with Klebsiella pneumonia ATCC 10031. In the KPD + CEQ-PLGA-MS and KPD + INJ groups, infected rats were intravenously injected with 12.5 mg/kg body weight CEQ-PLGA-MS and CEQ-INJ, respectively. RESULTS: Compared to CEQ-INJ treatment group, CEQ-PLGA-MS treatment further decreased the number of bacteria colonies (decreased to 1.94 lg CFU/g) in lung tissues and the levels of inflammatory cytokine including tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, IL-6, IL-4 (p < 0.05 or p < 0.01) in bronchoalveolar lavage fluid at 48 h. Consistently, a significant decreases of scores of inflammation severity were showed at 48 h in the KPD + CEQ-PLGA-MS treatment group, compared to the KPD + CEQ-INJ treatment group. CONCLUSION: Our results reveal that CEQ-PLGA-MS has the better therapeutic effect than CEQ-INJ for Klebsiella pneumonia lung infections in rats. The vehicle of CEQ-PLGA-MS as the promising alternatives to control the lung infections with the important pathogens.
Microdialysis Determination of Cefquinome Pharmacokinetics in Murine Thigh From Healthy, Neutropenic, and Actinobacillus pleuropneumoniae-Infected Mice.[Pubmed:30914957]
Front Pharmacol. 2019 Mar 12;10:249.
This study was aimed at applying microdialysis to explore Cefquinome pharmacokinetics in thigh and plasma of healthy, neutropenic, and Actinobacillus pleuropneumoniae-infected mice. The relative recoveries (RRs) were tested in vitro by dialysis and retrodialysis and in vivo by retrodialysis. ICR mice were randomly divided into four groups: H-40 (healthy mice receiving Cefquinome at 40 mg/kg), H-160, N-40 (neutropenic mice), and I-40 mg/kg (thigh infected-mice with A. pleuropneumoniae). After Cefquinome administration, plasma was collected by retro-orbital puncture and thigh dialysate was collected by using a microdialysis probe with Ringer's solution at a perfusion rate of 1.5 muL/min. Plasma and thigh dialysate samples were assessed by HPLC-MS/MS and analyzed by a non-compartment model. The mean in vivo recoveries in the thigh were 39.35, 38.59, and 37.29% for healthy, neutropenic, and infected mice, respectively. The mean plasma protein-binding level was 16.40% and was independent of drug concentrations. For all groups, the mean values of the free AUCinf in plasma were higher than those in murine thigh, while the elimination T 1/2beta for plasma were lower than those for murine thigh. Cefquinome penetration (AUCthigh/AUCplasma) from the plasma to thigh was 0.76, 0.88, 0.47, and 0.98 for H-40, N-40, I-40, and H-160 mg/kg, respectively. These results indicated that infection significantly affected Cefquinome pharmacokinetics in murine thigh. In conclusion, we successfully applied a microdialysis method to evaluate the pharmacokinetics of Cefquinome in murine thigh of healthy, neutropenic, and A. pleuropneumonia-infected mice and the pharmacokinetics of Cefquinome was obviously affected by infection in thigh.
A review on the characteristic, properties and analytical methods of cefquinome sulphate: ss-lactam veterinary drug.[Pubmed:30277168]
Infect Disord Drug Targets. 2018 Oct 1. pii: IDDT-EPUB-93320.
BACKGROUND: Chemotherapy as a science began within the 1st decade of the twentieth century with understanding of the principles of selective toxicity, the particular chemical relationships between microorganism pathogens and medicines, the event of drug resistance, and also the role of combined medical aid. OBJECTIVES: This review aims to highlight the characteristics, specifically the pharmacokinetic parameters, and the analytical methods reported in literature for the determination of Cefquinome, a fourth generation cephalosporineused to treat Gram-positive and Gram-negative caused infections. CONCLUSION: Analysis of such drugs, whether used for treatment of human or animal illness, is essential in understanding the bioavailability and therapeutic control which will ensure their activity and safety.
Selection of DNA aptamers and establishment of an effective aptasensor for highly sensitive detection of cefquinome residues in milk.[Pubmed:29872833]
Analyst. 2018 Jun 25;143(13):3202-3208.
Cefquinome (CFQ), which is a fourth-generation cephalosporin approved for veterinary use only, has been widely used for treating porcine or bovine respiratory infection, bovine mastitis and other diseases. However, the antibacterial effect of CFQ is based on the duration of drug concentration remaining in excess of the minimum inhibitory concentration in serum or tissues, thereby inevitably leading to CFQ residues with high levels in animal-sourced food. In this paper, four CFQ-specific ssDNA aptamers were selected via a magnetic bead-based systematic evolution of ligands by the exponential enrichment (SELEX) method. Aptamer W1 with the lowest dissociation constant (Kd) value of 40.13 +/- 22.11 nM was chosen for establishing a fluorescence aptasensor based on magnetic separation and release of molecular beacons for detection of CFQ residues. This aptasensor exhibited a high sensitivity toward CFQ with a limit of detection (LOD) of 0.09 ng mL-1 (linear range from 0.5 to 150 ng mL-1). Moreover, the present aptasensor also showed high selectivity against ampicillin and CFQ's structural analogs (i.e., cefpirome sulfate and cefixime). Finally, this aptasensor was used to detect CFQ in real spiked milk. The recovery rate of CFQ from spiked milk samples ranged from 96.6% to 103.2%. These results indicated that the developed aptasensor is a promising, highly sensitive and specific method for CFQ residue detection in animal-sourced food.
Histidine Metabolism and IGPD Play a Key Role in Cefquinome Inhibiting Biofilm Formation of Staphylococcus xylosus.[Pubmed:29675012]
Front Microbiol. 2018 Apr 5;9:665.
Staphylococcus xylosus (S. xylosus) is an AT-rich and coagulase-negative Staphylococcus (CNS). It is normally regarded as non-pathogenic, however, recent studies have demonstrated that it is related to human opportunistic infections and bovine mastitis. In addition, S. xylosus strains have the ability to form biofilm. Biofilms are also involved in chronic infections and antibiotic resistance, there are only a few reports about Cefquinome inhibiting S. xylosus biofilm formation and the protein targets of Cefquinome. In our study, we found that sub-MICs of Cefquinome were sufficient to inhibit biofilm formation. To investigate the potential protein targets of Cefquinome, we used iTRAQ for the analyses of cells at two different conditions: 1/2-MIC (0.125 mug/mL) Cefquinome treatment and no treatment. Using iTRAQ technique and KEGG database analysis, we found that proteins differently expression in histidine metabolism pathway may play a role in the process by which 1/2-MIC (0.125 mug/mL) Cefquinome inhibits S. xylosus biofilm formation. Interestingly, we found a sharply down-regulated enzyme [A0A068E9J3 imidazoleglycerol-phosphate dehydratase (IGPD)] involved in histidine metabolism pathway in Cefquinome-treated cells. We demonstrated the important role of IGPD in sub-MICs Cefquinome inhibiting biofilm formation of S. xylosus by gene (hisB) knockout, IGPD enzyme activity and histidine content assays. Thus, our data sheds light on important role of histidine metabolism in S. xylosus biofilm formation; especially, IGPD involved in histidine metabolism might play a crucial role in sub-MICs Cefquinome inhibition of biofilm formation of S. xylosus, and we propose IGPD as an attractive protein target of Cefquinome.
Cefquinome-loaded microsphere formulations against Klebsiella pneumonia infection during experimental infections.[Pubmed:29649952]
Drug Deliv. 2018 Nov;25(1):909-915.
The aim of this study was to prepare Cefquinome-loaded polylactic acid microspheres and to evaluate their in vitro and in vivo characteristics and pharmacodynamics for the therapy of pneumonia in a rat model. Microspheres were prepared using a 0.7 mm two-fluid nozzle spray drier in one step resulting in spherical and smooth microspheres of uniform size (9.8 +/- 3.6 mum). The encapsulation efficiency and drug loading of Cefquinome were 91.6 +/- 2.6% and 18.7 +/- 1.2%, respectively. In vitro release of Cefquinome from the microspheres was sustained for 36 h. Cefquinome-loaded polylactic acid microspheres as a drug delivery system was successful for clearing experimental Klebsiella pneumonia lung infections. A decrease in inflammatory cells and an inhibition of inflammatory cytokines TNF-alpha, IL-1beta and IL-8 after microspheres treatment was found. Changes in cytokine levels and types are secondary manifestations of drug bactericidal effects. Rats were considered to be microbiologically cured because the bacterial load was less than 100 CFU/g. These results also indicated that the spray-drying method of loading therapeutic drug into polylactic acid microspheres is a straightforward and safe method for lung-targeting therapy in animals.
A Comparison of Two Methods for the Preparation Cefquinome-Loaded Gelatin Microspheres for Lung Targeting.[Pubmed:29404707]
Pharm Res. 2018 Feb 5;35(2):43.
PURPOSE: The aim of this study was to prepare CEQ-loaded gelatin microspheres and compare two preparation methods, evaluate targeting to the lungs. METHODS: Gelatin microspheres containing CEQ were prepared by an emulsion cross-linking method (ECLM) and a spray-drying method (SDM) and were characterized in terms of morphology, size, drug-loading coefficient, encapsulation ratio and in vitro release. RESULTS: The microspheres prepared by ECLM gave a drug loading (DL) of 19.4 +/- 2.4% and an entrapment efficiency (EE) of 80.8 +/- 3.2%. The microspheres prepared by SDM resulted in a DL value of 20.8 +/- 2.7% and an EE of 95.3 +/- 3.8%. The average particle size of microspheres was 7-30 mum by both methods and both preparations sustained CEQ release for 36 h in the target tissue (lungs). The in vitro release profile of the microspheres matched the Korsmeyer-Peppas release pattern. In vivo studies identified the lung as the target tissue and the region of maximum CEQ release. Histopathological examination showed a partial lung inflammation that disappeared spontaneously as the microspheres were biodegraded. In general, the formulations were safe. CONCLUSION: The well-sustained CEQ release from the microspheres revealed its suitability as a drug delivery vehicle that minimized injury to healthy tissues while achieving the accumulation of therapeutic drug for lung targeting. The intravenous administration of CEQ gelatin microspheres prepared by SDM is of potential value in treating lung diseases in animals.


