Calcineurin Autoinhibitory PeptideCaMKII inhibitor CAS# 148067-21-4 |
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Fumonisin B1
Catalog No.:BCC2461
CAS No.:116355-83-0
- DL-AP3
Catalog No.:BCC2459
CAS No.:20263-06-3
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 148067-21-4 | SDF | Download SDF |
| PubChem ID | 16131045 | Appearance | Powder |
| Formula | C124H205N39O39S2 | M.Wt | 2930.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Autoinhibitory peptide 457-482 | ||
| Solubility | Soluble to 1 mg/ml in water | ||
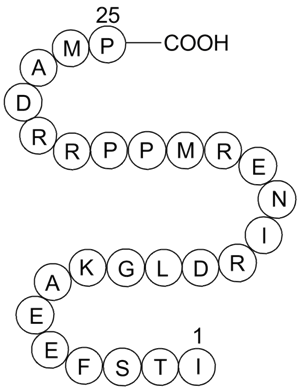
| Sequence | ITSFEEAKGLDRINERMPPRRDAMP | ||
| SMILES | CCC(C)C(C(=O)NC(C(C)O)C(=O)NC(CO)C(=O)NC(CC1=CC=CC=C1)C(=O)OC(=O)CCC(C(=O)NC(C)C(=O)NC(CCCCN)C(=O)NCC(=O)NC(CC(C)C)C(=O)OC(=O)CCC(C(=O)NC(CCCNC(=N)N)C(=O)NC(CCSC)C(=O)N2CCCC2C(=O)N3CCCC3C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(CC(=O)O)C(=O)NC(C)C(=O)NC(CCSC)C(=O)N4CCCC4C(=O)O)NC(=O)C(CC(=O)N)NC(=O)C(C(C)CC)NC(=O)C(CCCNC(=N)N)NC(=O)C(CC(=O)O)N)NC(=O)C(CCC(=O)O)N)N | ||
| Standard InChIKey | QKXOLPYOGCLSCP-TVQDNDAUSA-N | ||
| Standard InChI | InChI=1S/C124H205N39O39S2/c1-12-62(5)94(129)112(191)160-96(66(9)165)114(193)158-83(60-164)110(189)157-82(55-67-26-15-14-16-27-67)120(200)202-93(175)40-37-75(148-99(178)68(126)36-39-89(168)169)102(181)143-64(7)97(176)146-70(28-17-18-44-125)101(180)142-59-88(167)145-81(54-61(3)4)119(199)201-92(174)41-38-76(151-109(188)79(57-87(128)166)156-113(192)95(63(6)13-2)159-107(186)74(32-22-48-141-124(136)137)147-100(179)69(127)56-90(170)171)106(185)150-71(29-19-45-138-121(130)131)104(183)154-78(43-53-204-11)115(194)162-50-24-34-85(162)117(196)161-49-23-33-84(161)111(190)152-73(31-21-47-140-123(134)135)103(182)149-72(30-20-46-139-122(132)133)105(184)155-80(58-91(172)173)108(187)144-65(8)98(177)153-77(42-52-203-10)116(195)163-51-25-35-86(163)118(197)198/h14-16,26-27,61-66,68-86,94-96,164-165H,12-13,17-25,28-60,125-127,129H2,1-11H3,(H2,128,166)(H,142,180)(H,143,181)(H,144,187)(H,145,167)(H,146,176)(H,147,179)(H,148,178)(H,149,182)(H,150,185)(H,151,188)(H,152,190)(H,153,177)(H,154,183)(H,155,184)(H,156,192)(H,157,189)(H,158,193)(H,159,186)(H,160,191)(H,168,169)(H,170,171)(H,172,173)(H,197,198)(H4,130,131,138)(H4,132,133,139)(H4,134,135,140)(H4,136,137,141)/t62-,63-,64-,65-,66+,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,94-,95-,96-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of Ca2+-calmodulin-dependent protein phosphatase (calcineurin) (IC50 ~ 10 μM). Does not inhibit PP1, PP2A or CaM kinase II (IC50 > 100 mM). |

Calcineurin Autoinhibitory Peptide Dilution Calculator

Calcineurin Autoinhibitory Peptide Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Selective inhibitor of Ca2+-calmodulin-dependent protein phosphatase (calcineurin) (IC50 ~ 10 mM). Does not inhibit PP1, PP2A or CaM kinase II (IC50 > 100 mM).
- 25-Hydroxycycloart-23-en-3-one
Catalog No.:BCN1657
CAS No.:148044-47-7
- 1-(3-(1-Hydroxy-3-methylbutyl)-4-methoxyphenyl)ethan-1-one
Catalog No.:BCN7493
CAS No.:148044-44-4
- Doripenem
Catalog No.:BCC4094
CAS No.:148016-81-3
- Melphalan
Catalog No.:BCC2403
CAS No.:148-82-3
- Thiabendazole
Catalog No.:BCC3868
CAS No.:148-79-8
- Pilocarpin Nitrate
Catalog No.:BCC8234
CAS No.:148-72-1
- Beta-Tocopherol
Catalog No.:BCN6683
CAS No.:148-03-8
- Dinitolmide
Catalog No.:BCC8945
CAS No.:148-01-6
- Isokadsurenin D
Catalog No.:BCN6615
CAS No.:147976-35-0
- CA-074 Me
Catalog No.:BCC3649
CAS No.:147859-80-1
- Filic-3-en-25-al
Catalog No.:BCN6445
CAS No.:147850-78-0
- Niazinin
Catalog No.:BCN7623
CAS No.:147821-57-6
- Talc
Catalog No.:BCC4730
CAS No.:14807-96-6
- Fmoc-Lys(Dnp)-OH
Catalog No.:BCC3519
CAS No.:148083-64-1
- Ac-Lys(Fmoc)-OH
Catalog No.:BCC2679
CAS No.:148101-51-3
- trans-2-Tridecene-1,13-dioic acid
Catalog No.:BCN3667
CAS No.:14811-82-6
- (±)-Epibatidine
Catalog No.:BCC6750
CAS No.:148152-66-3
- UNC 0642
Catalog No.:BCC8014
CAS No.:1481677-78-4
- H-Dap-OH.HCl
Catalog No.:BCC3186
CAS No.:1482-97-9
- Secoisolariciresinol Diglucoside
Catalog No.:BCN1212
CAS No.:148244-82-0
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- (+)-Matairesinol
Catalog No.:BCN7021
CAS No.:148409-36-3
- Prion Protein 106-126 (human)
Catalog No.:BCC6027
CAS No.:148439-49-0
- L-732,138
Catalog No.:BCC6821
CAS No.:148451-96-1
Discovery of a latent calcineurin inhibitory peptide from its autoinhibitory domain by docking, dynamic simulation, and in vitro methods.[Pubmed:26111023]
J Biomol Struct Dyn. 2016 May;34(5):983-92.
Autoinhibitory domain (AID) of calcineurin (CN) was discovered two decades ago. Fewer investigations are reported to find out shortest possible peptide from the AID for CN inhibition. Hence, this study has focused on screening of nearly 150 peptide fragments derived from the AID using in silico method. Therefore, we have employed docking studies, aiming to analyze the best pose of AID-derived peptides on CN active site. We also analyzed binding free energy (DeltaG) of docked complex using molecular mechanics/generalized Born surface area (MM/GBSA). MM/GBSA predicts two short peptides P1 and P2 found to be lowest binding free energy. Two peptides exhibit better binding affinity with CN, suggests that the possible candidates for potential CN inhibition. Further, the stability of the docked complex was analyzed using molecular dynamic (MD) simulation. MD study shows that CNA:P2 is the most stable complex than CN A:P1 and CN A:AID. Besides, we have synthesized and purified P1 and P2 peptides over high performance liquid chromatography (HPLC) found to be 90.31% and 98.93% of purity, respectively. In addition, AID peptides were characterized over mass spectral analysis. Peptides were subjected to CN inhibitory assay using malachite green method. Where, P1 and P2 exhibit CN inhibition better than AID. In particular, shortest peptide P2 shows highest inhibitory activity than AID. Enzyme assay reveals CN inhibitory activity of P2 peptide is consistent within silico results. In silico and in vitro, results corroborated each other to confirm short peptide P2 can be used as a potential CN inhibitor.
Inhibition of excitatory neuronal cell death by cell-permeable calcineurin autoinhibitory peptide.[Pubmed:14622094]
J Neurochem. 2003 Dec;87(5):1145-51.
In glutamate-mediated excitatory neuronal cell death, immunosuppressants (FK506, Cys-A) are powerful agents that protect neurons from apoptosis. Immunosuppressants inhibit two types of enzyme, calcium/calmodulin-dependent protein phosphatase (calcineurin: CaN), and peptidyl-prolyl cis-trans-isomerase (PPIase) activity such as the FKBP family. In this study, we used a protein transduction approach to determine the functional role of CaN and to produce a potential therapeutic agent for glutamate-mediated neuronal cell death. We created a novel cell-permeable CaN autoinhibitory peptide using the 11 arginine protein transduction domain. This peptide was highly efficient at transducing into primary culture neurons, potently inhibited CaN phosphatase activities, and inhibited glutamate-mediated neuronal cell death. These results showed that CaN plays an important role in excitatory neuronal cell death and cell-permeable CaN autoinhibitory peptide could be a new drug to protect neurons from excitatory neuronal death.
Effect on protein phosphatase activity of peptide backbone modification and truncation of the autoinhibitory domain peptide of calcineurin.[Pubmed:8907505]
Int J Pept Protein Res. 1996 Jan-Feb;47(1-2):98-102.
Solid-phase synthesis of the autoinhibitory domain of calcineurin, CaN A467-491, also produced [aspartimide477]CaN A467-491 and [iso-Asp477]CaN467-491 when Boc-based chemistry was employed. In addition, the truncated peptide CaN A467-488 was obtained when Fmoc-based chemistry was employed. All four peptides proved to be effective inhibitors of protein phosphatase activity of calcineurin. The full-length peptide and the C-terminally truncated peptide (CaN467-488) were indistinguishable, with Ki values of 28 +/- 3 and 31 +/- 5 mu M, respectively. The internally modified peptides, [iso-Asp477]CaN A467-491 and [aspartimide477]-CaN A467-491, possessed lower inhibitory potencies (Ki values of 87 +/- 10 and 55 +/- 3 mu M, respectively).
Calcium regulation of calcineurin phosphatase activity by its B subunit and calmodulin. Role of the autoinhibitory domain.[Pubmed:7814394]
J Biol Chem. 1995 Jan 6;270(1):340-6.
Calcineurin (CaN) contains an autoinhibitory element (residues 457-482) 43 residues COOH-terminal of the calmodulin-binding domain (Hashimoto, Y., Perrino, B. A., and Soderling, T. R. (1990) J. Biol. Chem. 265, 1924-1927) that regulates the Ca(2+)-dependent activation of its phosphatase activity. Substitution of Arg476 and Arg477 or Asp467 to Ala in the autoinhibitory peptide 457-482 significantly decreased its inhibitory potency. CaN A subunits with these residues mutated to Ala were coexpressed with the Ca(2+)-binding B subunit using the baculovirus/Sf9 cell system. Kinetic analysis showed that although the purified mutants had no activity in the absence of calcium, they were less dependent than the wild-type enzyme on calcium and calmodulin for activity. To determine if additional autoinhibitory motifs were present in the COOH terminus of calcineurin, the A subunit was truncated at residues 457 or 420 and co-expressed with B subunit. The Vmax values of both truncation mutants with or without Ca2+ were increased relative to wild-type calcineurin. The increased Ca(2+)-independent activity of CaN420 relative to CaN457 indicates the presence of additional autoinhibitory element(s) within residues 420-457. CaN420 had similar high Vmax values with or without Ca2+, but the Km value for peptide substrate was increased 5-fold to 125 microM in the absence of Ca2+. The Km values of all the expressed calcineurin species were increased in the absence of Ca2+. The CaN A or CaN A420 subunits alone have low Vmax and high Km (115 microM) values even in the presence of Ca2+. These results indicate that 1) there are several autoinhibitory motifs between the CaM-binding domain and the COOH terminus that are relieved by Ca2+ binding to CaM and the B subunit, 2) Ca2+ binding to the B subunit also regulates enzyme activity by lowering the Km of the catalytic subunit for substrate, 3) binding of the B subunit is required for high Vmax values even after removal of the autoinhibitory domain. These results are consistent with synergistic activation of calcineurin by Ca2+ acting through both CaM and the B subunit.
The role of the autoinhibitory domain in differential metal ion activation of calmodulin-stimulated phosphatase.[Pubmed:8287965]
FEBS Lett. 1994 Jan 10;337(2):128-30.
Metal ion activators, Ni2+ and Mn2+, have been suggested to induce different conformations of calmodulin (CaM)-stimulated phosphatase. In the present study, an autoinhibitory domain previously implicated in the conformation transition of CaM stimulation of the phosphatase is shown to participate in defining the differential metal ion activation. A proteolytic derivative of the phosphatase deleted from the autoinhibitory domain displayed CaM-independent Mn(2+)-stimulated activity which was about 4-times that of the CaM-stimulated activity of the native enzyme. The Ni(2+)-stimulated activity of the derivative, on the other hand, retained slight CaM-dependence, and the CaM-stimulated activity was 90% of that of the native enzyme. A synthetic peptide corresponding to the autoinhibitory domain could inhibit the Mn(2+)-stimulated activity of the phosphatase derivative by 80%, but had little effect on the Ni(2+)-stimulated activity.
Identification of an autoinhibitory domain in calcineurin.[Pubmed:2153670]
J Biol Chem. 1990 Feb 5;265(4):1924-7.
The hypothesis that calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, contains an autoinhibitory domain was tested using synthetic peptides corresponding to regions of the carboxyl-terminus of calcineurin. Of the several peptides analyzed, one, containing residues I-T-S-F-E-E-A-K-G-L-D-R-I-N-E-R-M-P-P-R-R-D-A-M-P, gave complete inhibition of its protein phosphatase activity. Using [32P]myosin light chain as substrate an IC50 of about 10 microM was obtained with either native calcineurin, assayed in the presence of Ca2+/calmodulin, or with calcineurin subjected to partial proteolysis which converts it to a fully active phosphatase when assayed in the presence of [ethylenebis (oxyethylenenitrilo)]tetraacetic acid. With 50 mM p-nitrophenylphosphate as substrate an IC50 of about 40 microM was observed. Studies with overlapping peptides suggested that the sequence P-P-R-R-D-A-M-P was essential but not sufficient for the observed inhibition. Kinetic analysis indicated that the inhibition of phosphatase activity was not competitive with respect to [32P]myosin light chain. This peptide did not show significant inhibition of the catalytic subunits of protein phosphatases type I or type IIA or of Ca2+/calmodulin-dependent protein kinase II. These results indicate that amino acids within this sequence of calcineurin constitute a unique autoinhibitory domain which interacts with the active site and is responsible for the low basal phosphatase activity in the absence of Ca2+/calmodulin.


