NVP-BEP800Oral Hsp90β inhibitor, novel, fully synthetic CAS# 847559-80-2 |
- PDK1 inhibitor
Catalog No.:BCC1843
CAS No.:1001409-50-2
- GSK2334470
Catalog No.:BCC4982
CAS No.:1227911-45-6
- BX-912
Catalog No.:BCC1250
CAS No.:702674-56-4
- OSU-03012 (AR-12)
Catalog No.:BCC1255
CAS No.:742112-33-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 847559-80-2 | SDF | Download SDF |
| PubChem ID | 25210273 | Appearance | Powder |
| Formula | C21H23Cl2N5O2S | M.Wt | 480.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | VER-82576 | ||
| Solubility | DMSO : 6.2 mg/mL (12.91 mM; Need ultrasonic) | ||
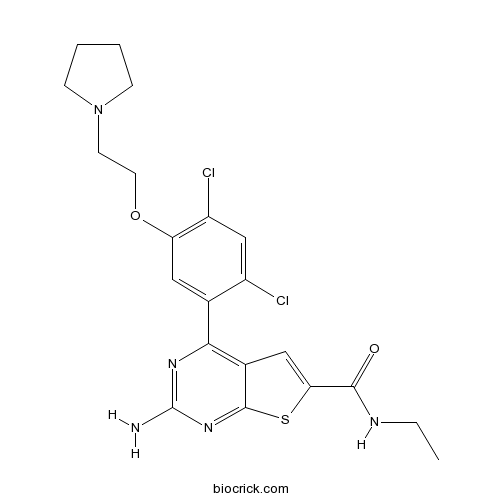
| Chemical Name | 2-amino-4-[2,4-dichloro-5-(2-pyrrolidin-1-ylethoxy)phenyl]-N-ethylthieno[2,3-d]pyrimidine-6-carboxamide | ||
| SMILES | CCNC(=O)C1=CC2=C(N=C(N=C2S1)N)C3=CC(=C(C=C3Cl)Cl)OCCN4CCCC4 | ||
| Standard InChIKey | WJUNQSYQHHIVFX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H23Cl2N5O2S/c1-2-25-19(29)17-10-13-18(26-21(24)27-20(13)31-17)12-9-16(15(23)11-14(12)22)30-8-7-28-5-3-4-6-28/h9-11H,2-8H2,1H3,(H,25,29)(H2,24,26,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | NVP-BEP800 is a novel, fully synthetic, oral inhibitor of Hsp90β with IC50 value of 58 nM. | |||||
| Targets | Hsp90β | |||||
| IC50 | 58 nM | |||||

NVP-BEP800 Dilution Calculator

NVP-BEP800 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0816 mL | 10.408 mL | 20.816 mL | 41.632 mL | 52.04 mL |
| 5 mM | 0.4163 mL | 2.0816 mL | 4.1632 mL | 8.3264 mL | 10.408 mL |
| 10 mM | 0.2082 mL | 1.0408 mL | 2.0816 mL | 4.1632 mL | 5.204 mL |
| 50 mM | 0.0416 mL | 0.2082 mL | 0.4163 mL | 0.8326 mL | 1.0408 mL |
| 100 mM | 0.0208 mL | 0.1041 mL | 0.2082 mL | 0.4163 mL | 0.5204 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NVP-BEP800 is a fully synthetic, orally bioavailable inhibitor of Hsp90 with IC50 value of 58nM [1].
NVP-BEP800 binds to the N-terminal ATP-binding pocket of Hsp90. In a competitive binding fluorescence polarization assay, NVP-BEP800 inhibits Hsp90β with IC50 value of 58nM. And to other 20 protein kinases, NVP-BEP800 shows a IC50 of >10μM. In BT-474 cells and A375 cells, NVP-BEP800 causes the Hsp90-p23 dissociation and client protein degradation (ErbB2) as well as the reduction of client protein phosphorylation (phospho-Akt). Degradation of these oncogenic client proteins results in tumor cell growth arrest and death. NVP-BEP800 inhibits proliferation of tumor cells with an average GI50 of 245nM. And in 46 primary human tumors including small cell lung, mammary cancer and melanoma, the mean IC50 is 750nM. Additionally, treatment of NVP-BEP800 induces apoptosis in human breast cancer cell lines. The antitumor efficacy of NVP-BEP800 is also observed with a dose of 15 or 30 mg/kg/d in A375 xenograft-bearing mice as well as in BT-474 breast cancer xenografts [1].
References:
[1] Massey AJ, Schoepfer J, Brough PA, Brueggen J, Chène P, Drysdale MJ, Pfaar U, Radimerski T, Ruetz S, Schweitzer A, Wood M, Garcia-Echeverria C, Jensen MR. Preclinical antitumor activity of the orally available heat shock protein 90 inhibitor NVP-BEP800. Mol Cancer Ther. 2010 Apr;9(4):906-19.
- SHA 68
Catalog No.:BCC6210
CAS No.:847553-89-3
- CEP-18770
Catalog No.:BCC2093
CAS No.:847499-27-8
- Eriocalyxin B
Catalog No.:BCN4390
CAS No.:84745-95-9
- Qingyangshengenin
Catalog No.:BCN4389
CAS No.:84745-94-8
- Calceolarioside A
Catalog No.:BCN5347
CAS No.:84744-28-5
- UVI 3003
Catalog No.:BCC7638
CAS No.:847239-17-2
- Arglabin
Catalog No.:BCC5299
CAS No.:84692-91-1
- Astragaloside IV
Catalog No.:BCN5960
CAS No.:84687-43-4
- Astragaloside III
Catalog No.:BCN5963
CAS No.:84687-42-3
- Astragaloside I
Catalog No.:BCN5961
CAS No.:84680-75-1
- 3-O-(2'E,4'Z-Decadienoyl)ingenol
Catalog No.:BCN3767
CAS No.:84680-59-1
- Enalaprilat Dihydrate
Catalog No.:BCC5009
CAS No.:84680-54-6
- ICG 001
Catalog No.:BCC3632
CAS No.:847591-62-2
- Tasumatrol L
Catalog No.:BCN6955
CAS No.:847835-17-0
- S 32212 hydrochloride
Catalog No.:BCC6208
CAS No.:847871-78-7
- Lenalidomide hemihydrate
Catalog No.:BCC4198
CAS No.:847871-99-2
- RO4929097
Catalog No.:BCC2089
CAS No.:847925-91-1
- Lck Inhibitor
Catalog No.:BCC1689
CAS No.:847950-09-8
- 5-Epilithospermoside
Catalog No.:BCN4392
CAS No.:84799-31-5
- threo-1-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCN1331
CAS No.:848031-94-7
- 3,4-Dimethoxyphenyl beta-D-glucoside
Catalog No.:BCN4393
CAS No.:84812-00-0
- HKI 357
Catalog No.:BCC6046
CAS No.:848133-17-5
- Alvelestat
Catalog No.:BCC4058
CAS No.:848141-11-7
- EX-527 S-enantiomer
Catalog No.:BCC5594
CAS No.:848193-68-0
Hsp90 Inhibitors NVP-AUY922 and NVP-BEP800 May Exert a Significant Radiosensitization on Tumor Cells along with a Cell Type-Specific Cytotoxicity.[Pubmed:23066444]
Transl Oncol. 2012 Oct;5(5):356-69. Epub 2012 Oct 1.
Targeting heat shock protein 90 (Hsp90) provides a promising therapeutic approach to enhance the sensitivity of tumor cells to ionizing radiation (IR). To explore the impact of scheduling drug-IR administration, in the present study, we analyzed the response of lung carcinoma A549 and glioblastoma SNB19 cells to simultaneous drug-IR treatment followed by a long-term drug administration. Cellular response was evaluated at different time intervals after IR-alone, drug-alone, or combined drug-IR treatments by colony counts and expression profiles of Hsp90 and its clients, along with several apoptotic markers and cell cycle-related proteins, as well as by IR-drug-induced cell cycle arrest, DNA damage, and repair. A short 30-minute exposure to either Hsp90 inhibitor did not affect the radiosensitivity of both tumor cell lines. Increasing the duration of post-IR-drug treatment progressively enhanced the sensitivity of SNB19 cells to IR. In contrast, the response of A549 cells to drug-IR combination was largely determined by the cytotoxic effects of both drugs without radiosensitization. Combined drug-IR treatment induced more severe DNA damage in both tumor cell lines than each treatment alone and also protracted the kinetics of DNA damage repair in SNB19 cells. In addition to large cell cycle disturbances, drug-IR treatment also caused depletion of the antiapoptotic proteins Akt and Raf-1 in both cell lines, along with a decrease of survivin in A549 cells in case of NVP-AUY922. The data show that simultaneous Hsp90 inhibition and irradiation may induce cell type-specific radiosensitization as well as cytotoxicity against tumor cells.
Irradiation facilitates the inhibitory effect of the heat shock protein 90 inhibitor NVP-BEP800 on the proliferation of malignant glioblastoma cells through attenuation of the upregulation of heat shock protein 70.[Pubmed:25120620]
Exp Ther Med. 2014 Sep;8(3):893-898.
The present study aimed to investigate the effect of NVP-BEP800, a novel heat shock protein (Hsp) 90 inhibitor of the 2-aminothieno[2,3-d]pyrimidine class, in combination with radiation on glioblastoma cells. T98G human glioblastoma cells were treated with dimethyl sulfoxide (DMSO), NVP-BEP800, NVP-BEP800 in combination with X-ray irradiation (10 Gy, 20 min), or X-ray irradiation only, and cultured for 40 h. Cell viability was measured upon completion of the treatments. In addition, apoptosis was measured and immunoblot analysis was performed to analyze the expression levels of cellular protein inhibitory kappaB kinase beta (IKKbeta). The combined treatment with NVP-BEP800 and X-ray irradiation resulted in the synergistic destruction of malignant cells. Furthermore, NVP-BEP800 significantly induced apoptosis in the human glioblastoma cells. The immunoblot analysis data indicated that NVP-BEP800 markedly reduced the expression level of IKKbeta. The results also revealed that X-ray irradiation significantly attenuated the increase in the level of Hsp70 in cells treated with NVP-BEP800. Since elevated levels of Hsp70 are associated with drug resistance induced by Hsp90 inhibitors, the effects of X-ray irradiation on Hsp70 levels may be associated with the enhanced effect on cells of the presence of irradiation. The results of the current study suggest that irradiation enhances the inhibitory effect of NVP-BEP800 on the proliferation of malignant glioblastoma cells by downregulating the expression level of cellular signaling protein IKKbeta and attenuating the upregulation of Hsp70 that is induced by NVP-BEP800.
Novel HSP90 inhibitors, NVP-AUY922 and NVP-BEP800, radiosensitise tumour cells through cell-cycle impairment, increased DNA damage and repair protraction.[Pubmed:20502461]
Br J Cancer. 2010 May 25;102(11):1578-91.
BACKGROUND: Heat-shock protein 90 (Hsp90) has a crucial role in both the stabilisation and regulation of various proteins, including those related to radioresistance. Inhibition of Hsp90 may therefore provide a strategy for enhancing the radiosensitivity of tumour cells. This study explores the responses of four tumour cell lines (A549, GaMG, HT 1080 and SNB19) to combined treatment with ionising radiation (IR) and two novel inhibitors of Hsp90, NVP-AUY922 and NVP-BEP800. The techniques used included cell and colony counts, expression of Hsp90, Hsp70, Akt, survivin, cleaved caspase 3, p53, cell-cycle progression and associated proteins. DNA damage was analysed by histone gammaH2AX and Comet assays. RESULTS: We found that NVP-AUY922 and NVP-BEP800 enhanced radiosensitivity in all tested cell lines. In contrast, only two cell lines (HT 1080 and GaMG) exhibited an increased rate of apoptosis after drug pretreatment, as revealed by western blot. In all tested cell lines, the expression of histone gammaH2AX, a marker of DNA double-strand breaks, after combined drug-IR treatment was higher and its decay rate was slower than those after each single treatment modality. Drug-IR treatment also resulted in impaired cell-cycle progression, as indicated by S-phase depletion and G2/M arrest. In addition, the cell cycle-associated proteins, Cdk1 and Cdk4, were downregulated after Hsp90 inhibition. INTERPRETATION: These findings show that the novel inhibitors of Hsp90 can radiosensitise tumour cell lines of different entities through destabilisation and depletion of several Hsp90 client proteins, thus causing the depletion of S phase and G2/M arrest, increased DNA damage and repair protraction and, to some extent, apoptosis. The results might have important implications for the radiotherapy of solid tumours.
Hsp90 inhibition by NVP-AUY922 and NVP-BEP800 decreases migration and invasion of irradiated normoxic and hypoxic tumor cell lines.[Pubmed:23340178]
Cancer Lett. 2013 May 1;331(2):200-10.
This study explores the impact of Hsp90 inhibitors NVP-AUY922 and NVP-BEP800 in combination with ionizing radiation (IR) on the migration and invasion of lung carcinoma A549 and glioblastoma SNB19 cells, under normoxia or hypoxia. Independent of oxygen concentration, both drugs decreased the migration and invasion rates of non-irradiated tumor cells. Combined drug-IR treatment under hypoxia inhibited cell invasion to a greater extent than did each treatment alone. Decreased migration of cells correlated with altered expression of several matrix-associated proteins (FAK/p-FAK, Erk2, RhoA) and impaired F-actin modulation. The anti-metastatic efficacy of the Hsp90 inhibitors could be useful in combinational therapies of cancer.


