BI6727 (Volasertib)Plk inhibitor,highly potent CAS# 755038-65-4 |
- MLN0905
Catalog No.:BCC3961
CAS No.:1228960-69-7
- Rigosertib
Catalog No.:BCC4296
CAS No.:592542-59-1
- GW843682X
Catalog No.:BCC1614
CAS No.:660868-91-7
- BI 2536
Catalog No.:BCC2081
CAS No.:755038-02-9
- GSK461364
Catalog No.:BCC3788
CAS No.:929095-18-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 755038-65-4 | SDF | Download SDF |
| PubChem ID | 10461508 | Appearance | Powder |
| Formula | C34H50N8O3 | M.Wt | 618.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BI 6727 | ||
| Solubility | DMSO : 50 mg/mL (80.80 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
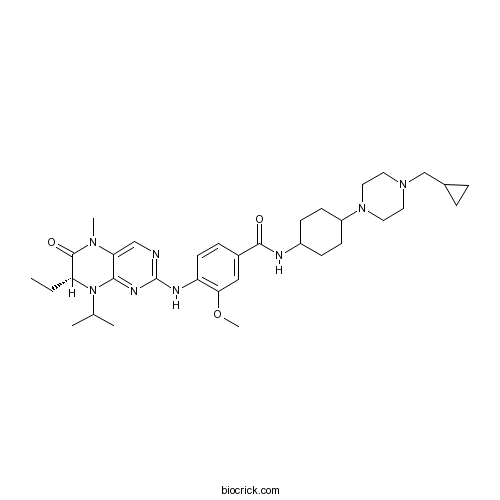
| Chemical Name | N-[4-[4-(cyclopropylmethyl)piperazin-1-yl]cyclohexyl]-4-[[(7R)-7-ethyl-5-methyl-6-oxo-8-propan-2-yl-7H-pteridin-2-yl]amino]-3-methoxybenzamide | ||
| SMILES | CCC1C(=O)N(C2=CN=C(N=C2N1C(C)C)NC3=C(C=C(C=C3)C(=O)NC4CCC(CC4)N5CCN(CC5)CC6CC6)OC)C | ||
| Standard InChIKey | SXNJFOWDRLKDSF-XKHVUIRMSA-N | ||
| Standard InChI | InChI=1S/C34H50N8O3/c1-6-28-33(44)39(4)29-20-35-34(38-31(29)42(28)22(2)3)37-27-14-9-24(19-30(27)45-5)32(43)36-25-10-12-26(13-11-25)41-17-15-40(16-18-41)21-23-7-8-23/h9,14,19-20,22-23,25-26,28H,6-8,10-13,15-18,21H2,1-5H3,(H,36,43)(H,35,37,38)/t25?,26?,28-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BI6727 is a high potent inhibitor of Polo-like kinase with IC50 value of 0.87 nM. | |||||
| Targets | Polo-like kinase | |||||
| IC50 | 0.87 nM | |||||

BI6727 (Volasertib) Dilution Calculator

BI6727 (Volasertib) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.616 mL | 8.0798 mL | 16.1595 mL | 32.3191 mL | 40.3988 mL |
| 5 mM | 0.3232 mL | 1.616 mL | 3.2319 mL | 6.4638 mL | 8.0798 mL |
| 10 mM | 0.1616 mL | 0.808 mL | 1.616 mL | 3.2319 mL | 4.0399 mL |
| 50 mM | 0.0323 mL | 0.1616 mL | 0.3232 mL | 0.6464 mL | 0.808 mL |
| 100 mM | 0.0162 mL | 0.0808 mL | 0.1616 mL | 0.3232 mL | 0.404 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BI6727 (Volasertib) is a selective inhibitor of Plk1, Plk2, and Plk3 with IC50 value of 0.87, 5 and 56 nM/L, respectively [1].
Polo-like kinase 1 (Plk1) is an early trigger for G2/M phase transition and is over-expressed in a variety of cancers for that being regarded as a promising target for cancer treatment [1].
BI6727 (Volasertib) is a potent Plk1 inhibitor and is regarded as a promising drug for multiple cancers in clinic. When tested with NB TICs and normal human pediatric SKPs (neural crest-like stem cells), BI6727 (Volasertib) treatment inhibits NB TICs with EC50 value of 21 nM/L and 2.8 μM/L on SKPs and decresed TIC survival [2]. It has been reported that BI6727 (Volasertib) inhibited proliferation of multiple cell lines, including HCT 116(caicinomas of the colon), NCI-H460 (lung cancer), BRO (melanoma), GRANTA (hematopoitic cancers) with EC50 value of 23 nmol/l, 21 nmol/l, 11 nmol/l, 15 nmol/l, respectively [1] [3].
In nude mice model with HCT 16 cells (colon carcinoma) subcutaneous xenograft, oral administration of BI6727 (Volasertib) delays tumor growth, decreased tumor size and induced tumor regression by increasing the mitotic index and apoptosis. And the same results were achieved when tested with NCI-H4660 (non-small cell lung carcinoma), xenograft model [1].
References:
[1]. Rudolph, D., et al., BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res, 2009. 15(9): p. 3094-102.
[2]. Grinshtein, N., et al., Small molecule kinase inhibitor screen identifies polo-like kinase 1 as a target for neuroblastoma tumor-initiating cells. Cancer Res, 2011. 71(4): p. 1385-95.
[3]. Munch, C., et al., Therapeutic polo-like kinase 1 inhibition results in mitotic arrest and subsequent cell death of blasts in the bone marrow of AML patients and has similar effects in non-neoplastic cell lines. Leuk Res, 2015. 39(4): p. 462-70.
- BI 2536
Catalog No.:BCC2081
CAS No.:755038-02-9
- Regorafenib
Catalog No.:BCC3646
CAS No.:755037-03-7
- N-Methylnuciferine
Catalog No.:BCN3971
CAS No.:754919-24-9
- CGP 37157
Catalog No.:BCC6943
CAS No.:75450-34-9
- Moxonidine
Catalog No.:BCC2142
CAS No.:75438-57-2
- EHT 1864
Catalog No.:BCC6075
CAS No.:754240-09-0
- Boc-Asp(OBzl)-OH
Catalog No.:BCC2608
CAS No.:7536-58-5
- Boc-Asn-OH
Catalog No.:BCC3071
CAS No.:7536-55-2
- Indacaterol Maleate
Catalog No.:BCC4358
CAS No.:753498-25-8
- Lovastatin
Catalog No.:BCN1060
CAS No.:75330-75-5
- H-Leucinol
Catalog No.:BCC2725
CAS No.:7533-40-6
- H-Pro-NH2
Catalog No.:BCC3018
CAS No.:7531-52-4
- Flupirtine maleate
Catalog No.:BCC4456
CAS No.:75507-68-5
- Cedrin
Catalog No.:BCN4748
CAS No.:75513-81-4
- Nilvadipine
Catalog No.:BCC3799
CAS No.:75530-68-6
- Moxonidine hydrochloride
Catalog No.:BCC5163
CAS No.:75536-04-8
- Dencichin
Catalog No.:BCN2555
CAS No.:7554-90-7
- Ingenol 3-Angelate
Catalog No.:BCN2961
CAS No.:75567-37-2
- 20-Deoxyingenol 3-angelate
Catalog No.:BCN6642
CAS No.:75567-38-3
- Sodium phosphate dibasic
Catalog No.:BCC7585
CAS No.:7558-79-4
- Sodium phosphate monobasic
Catalog No.:BCC8033
CAS No.:7558-80-7
- alpha-Tocopherolquinone
Catalog No.:BCN4305
CAS No.:7559-04-8
- Kaerophyllin
Catalog No.:BCN4304
CAS No.:75590-33-9
- Methyl 3,4,5-trimethoxycinnamate
Catalog No.:BCN4589
CAS No.:7560-49-8
Pharmaceutically inhibiting polo-like kinase 1 exerts a broad anti-tumour activity in retinoblastoma cell lines.[Pubmed:27647547]
Clin Exp Ophthalmol. 2017 Apr;45(3):288-296.
BACKGROUND: Retinoblastoma is the most common malignant cancer of the eye in children. Although metastatic retinoblastoma is rare, cure rates for this advanced disease remain below 50%. High-level polo-like kinase 1 expression in retinoblastomas has previously been shown to be correlated with adverse outcome parameters. Polo-like kinase 1 is a serine/threonine kinase involved in cell cycle regulation at the G2/M transition. Polo-like kinase 1 inhibition has been demonstrated to have anti-tumour effects in preclinical models of several paediatric tumours. Here, we assessed its efficacy against retinoblastoma cell lines. METHODS: Expression of polo-like kinase 1 was determined in a panel of retinoblastoma cell lines by polymerase chain reaction and western blot analysis. We analysed viability (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT assay), proliferation (5-bromo-2'-deoxyuridine enzyme-linked immunosorbent assay), cell cycle progression (propidium iodid staining) and apoptosis (cell death enzyme-linked immunosorbent assay) in three retinoblastoma cell lines after treatment with two adenosine triphosphate-competitive polo-like kinase 1 inhibitors, BI6727 or GSK461364. Activation of polo-like kinase 1 downstream signalling components including TP53 were assessed. RESULTS: Treatment of retinoblastoma cells with either BI6727 or GSK461364 reduced cell viability and proliferative capacity and induced both cell cycle arrest and apoptosis. Polo-like kinase 1 inhibition also induced the p53 signalling pathway. Analysis of key players in cell cycle control revealed that low nanomolar concentrations of either polo-like kinase 1 inhibitor upregulated cyclin B1 and increased activated cyclin-dependent kinase 1 (phosphorylated at Y15) in retinoblastoma cell lines. CONCLUSIONS: These preclinical data indicate that polo-like kinase 1 inhibitors could be useful as components in rationally designed chemotherapy protocols to treat patients with metastasized retinoblastoma in early phase clinical trials.
Polo-like Kinase 1 as a potential therapeutic target in Diffuse Intrinsic Pontine Glioma.[Pubmed:27538997]
BMC Cancer. 2016 Aug 18;16:647.
BACKGROUND: Diffuse intrinsic pontine gliomas (DIPGs) are highly aggressive, fatal, childhood tumors that arise in the brainstem. DIPGs have no effective treatment, and their location and diffuse nature render them inoperable. Radiation therapy remains the only standard of care for this devastating disease. New therapeutic targets are needed to develop novel therapy for DIPG. METHODS: We examined the expression of PLK1 mRNA in DIPG tumor samples through microarray analysis and found it to be up regulated versus normal pons. Using the DIPG tumor cells, we inhibited PLK1 using a clinically relevant specific inhibitor BI 6727 and evaluated the effects on, proliferation, apoptosis, induction of DNA damage and radio sensitization of the DIPG tumor cells. RESULTS: Treatment of DIPG cell lines with BI 6727, a new generation, highly selective inhibitor of PLK1, resulted in decreased cell proliferation and a marked increase in cellular apoptosis. Cell cycle analysis showed a significant arrest in G2-M phase and a substantial increase in cell death. Treatment also resulted in an increased gammaH2AX expression, indicating induction of DNA damage. PLK1 inhibition resulted in radiosensitization of DIPG cells. CONCLUSION: These findings suggest that targeting PLK1 with small-molecule inhibitors, in combination with radiation therapy, will hold a novel strategy in the treatment of DIPG that warrants further investigation.
Polo-like Kinase-1 Regulates Myc Stabilization and Activates a Feedforward Circuit Promoting Tumor Cell Survival.[Pubmed:27773673]
Mol Cell. 2016 Nov 3;64(3):493-506.
MYCN amplification in human cancers predicts poor prognosis and resistance to therapy. However, pharmacological strategies that directly target N-Myc, the protein encoded by MYCN, remain elusive. Here, we identify a molecular mechanism responsible for reciprocal activation between Polo-like kinase-1 (PLK1) and N-Myc. PLK1 specifically binds to the SCF(Fbw7) ubiquitin ligase, phosphorylates it, and promotes its autopolyubiquitination and proteasomal degradation, counteracting Fbw7-mediated degradation of N-Myc and additional substrates, including cyclin E and Mcl1. Stabilized N-Myc in turn directly activates PLK1 transcription, constituting a positive feedforward regulatory loop that reinforces Myc-regulated oncogenic programs. Inhibitors of PLK1 preferentially induce potent apoptosis of MYCN-amplified tumor cells from neuroblastoma and small cell lung cancer and synergistically potentiate the therapeutic efficacies of Bcl2 antagonists. These findings reveal a PLK1-Fbw7-Myc signaling circuit that underlies tumorigenesis and validate PLK1 inhibitors, alone or with Bcl2 antagonists, as potential effective therapeutics for MYC-overexpressing cancers.
Bcl-2 degradation is an additional pro-apoptotic effect of polo-like kinase inhibition in cholangiocarcinoma cells.[Pubmed:28652654]
World J Gastroenterol. 2017 Jun 14;23(22):4007-4015.
AIM: To examine the influence on apoptotic mechanisms following inhibition of polo-like kinases as therapeutically approach for cholangiocellular cancer treatment. METHODS: As most cholangiocarcinomas are chemotherapy-resistant due to mechanisms preventing tumor cell death, we investigated the effect of Cisplatin on cholangiocellular carcinoma (CCA) cell lines KMCH-1 and Mz-Ch-1. Polo-like kinases (PLK) are important regulators of the cell cycle and their inhibition is discussed as a potential therapy while PLK inhibition can regulate apoptotic mediators. Here, cells were treated with PLK inhibitor BI6727 (Volasertib), Cisplatin, and in combination of both compounds. Cell viability was assessed by MTT; apoptosis was measured by DAPI staining and caspase-3/-7 assay. Western blot and qRT-PCR were used to measure expression levels of apoptosis-related molecules Bax and Bcl-2. RESULTS: The cell viability in the CCA cell lines KMCH-1 and Mz-Ch-1 was reduced in all treatment conditions compared to vehicle-treated cells. Co-treatment with BI6727 and cisplatin could even enhance the cytotoxic effect of cisplatin single treatment. Thus, co-treatment of cisplatin with BI6727 could slightly enhance the cytotoxic effect of the cisplatin in both cell lines whereas there was evidence of increased apoptosis induction solely in Mz-Ch-1 as compared to KMCH-1. Moreover, PLK inhibition decreases protein levels of Bcl-2; an effect that can be reversed by the proteasomal degradation inhibitor MG-132. In contrast, protein levels of Bax were not found to be altered by PLK inhibition. These findings indicate that cytotoxic effects of Cisplatin in Mz-Ch-1 cells can be enhanced by cotreatment with BI6727. CONCLUSION: In conclusion, BI6727 treatment can sensitize CCA cells to cisplatin-induced apoptosis with proteasomal Bcl-2 degradation as an additional pro-apoptotic effect.
Spotlight on Volasertib: Preclinical and Clinical Evaluation of a Promising Plk1 Inhibitor.[Pubmed:27140825]
Med Res Rev. 2016 Jul;36(4):749-86.
Considering the important side effects of conventional microtubule targeting agents, more and more research focuses on regulatory proteins for the development of mitosis-specific agents. Polo-like kinase 1 (Plk1), a master regulator of several cell cycle events, has arisen as an intriguing target in this research field. The observed overexpression of Plk1 in a broad range of human malignancies has given rise to the development of several potent and specific small molecule inhibitors targeting the kinase. In this review, we focus on volasertib (BI6727), the lead agent in category of Plk1 inhibitors at the moment. Numerous preclinical experiments have demonstrated that BI6727 is highly active across a variety of carcinoma cell lines, and the inhibitor has been reported to induce tumor regression in several xenograft models. Moreover, volasertib has shown clinical efficacy in multiple tumor types. As a result, Food and Drug Administration (FDA) has recently awarded volasertib the Breakthrough Therapy status after significant benefit was observed in acute myeloid leukemia (AML) patients treated with the Plk1 inhibitor. Here, we discuss both preclinical and clinical data available for volasertib administered as monotherapy or in combination with other anticancer therapies in a broad range of tumor types.


