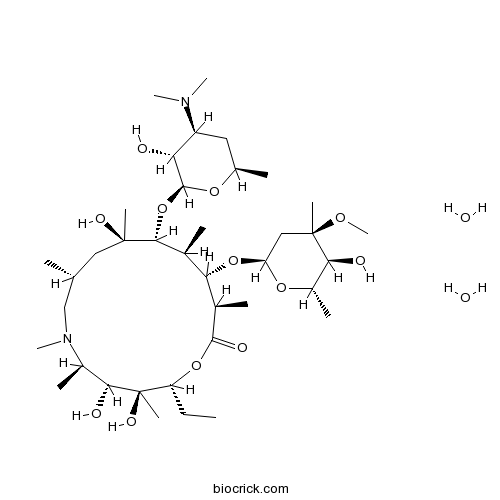
Azithromycin DihydrateCAS# 117772-70-0 |
- Nateglinide
Catalog No.:BCC5005
CAS No.:105816-04-4
- ML133 HCl
Catalog No.:BCC5006
CAS No.:1222781-70-5
- Dronedarone
Catalog No.:BCN2176
CAS No.:141626-36-0
- Gliclazide
Catalog No.:BCC5002
CAS No.:21187-98-4
- Tolbutamide
Catalog No.:BCC5001
CAS No.:64-77-7
- Nicorandil
Catalog No.:BCC5004
CAS No.:65141-46-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 117772-70-0 | SDF | Download SDF |
| PubChem ID | 3033819 | Appearance | Powder |
| Formula | C38H76N2O14 | M.Wt | 785.02 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CP-62993 dihydrate | ||
| Solubility | Soluble to 100 mg/mL (127.38 mM) in DMSO | ||
| Chemical Name | (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-11-[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one;dihydrate | ||
| SMILES | CCC1C(C(C(N(CC(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)OC)C)OC3C(C(CC(O3)C)N(C)C)O)(C)O)C)C)C)O)(C)O.O.O | ||
| Standard InChIKey | SRMPHJKQVUDLQE-KUJJYQHYSA-N | ||
| Standard InChI | InChI=1S/C38H72N2O12.2H2O/c1-15-27-38(10,46)31(42)24(6)40(13)19-20(2)17-36(8,45)33(52-35-29(41)26(39(11)12)16-21(3)48-35)22(4)30(23(5)34(44)50-27)51-28-18-37(9,47-14)32(43)25(7)49-28;;/h20-33,35,41-43,45-46H,15-19H2,1-14H3;2*1H2/t20-,21-,22+,23-,24-,25+,26+,27-,28+,29-,30+,31-,32+,33-,35+,36-,37-,38-;;/m1../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Azithromycin hydrate is a macrolide antibiotic useful for the treatment of a number of bacterial infections.In Vitro:Azithromycin (2 μM) augments rhinovirus-induced IFNβ expression in primary bronchial epithelial cells from asthmatics, which is associated with over-expression of RIG-I like receptors and repression of viral replication. Knockdown of MDA5, but not knockdown of RIG-I, diminishes azithromycin (2 μM)-enhanced viral-induced IFNβ expression in asthmatic primary bronchial epithelial cells[1]. Azithromycin specifically reduces MMP-9 mRNA and protein levels without affecting NF-κB in endotoxin-challenged monocytic THP-1 cells[2].In Vivo:Azithromycin (50 mg/kg) has no effect on bronchoalveolar lavage inflammatory parameters and LDH levels in a mouse model of asthma exacerbation. Azithromycin induces neither general inflammatory parameters nor LDH release in a mouse model of asthma exacerbation, and augments expression of interferon-stimulated genes and the pattern recognition receptor MDA5 but not RIG-I in exacerbating mice[1]. References: | |||||

Azithromycin Dihydrate Dilution Calculator

Azithromycin Dihydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2739 mL | 6.3693 mL | 12.7385 mL | 25.4771 mL | 31.8463 mL |
| 5 mM | 0.2548 mL | 1.2739 mL | 2.5477 mL | 5.0954 mL | 6.3693 mL |
| 10 mM | 0.1274 mL | 0.6369 mL | 1.2739 mL | 2.5477 mL | 3.1846 mL |
| 50 mM | 0.0255 mL | 0.1274 mL | 0.2548 mL | 0.5095 mL | 0.6369 mL |
| 100 mM | 0.0127 mL | 0.0637 mL | 0.1274 mL | 0.2548 mL | 0.3185 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Azithromycin Dihydrate is an acid stable orally administered macrolide antimicrobial drug, structurally related to erythromycin.
- CGH 2466 dihydrochloride
Catalog No.:BCC7338
CAS No.:1177618-54-0
- SMANT hydrochloride
Catalog No.:BCC6254
CAS No.:1177600-74-6
- N20C hydrochloride
Catalog No.:BCC7292
CAS No.:1177583-87-7
- Forsythoside I
Catalog No.:BCN6430
CAS No.:1177581-50-8
- Felbamate hydrate
Catalog No.:BCC4160
CAS No.:1177501-39-1
- (R)-(+)-Blebbistatin
Catalog No.:BCC7195
CAS No.:1177356-70-5
- CKI 7 dihydrochloride
Catalog No.:BCC5614
CAS No.:1177141-67-1
- Doramectin
Catalog No.:BCC1536
CAS No.:117704-25-3
- Dexamethasone acetate
Catalog No.:BCC4775
CAS No.:1177-87-3
- Laxogenin
Catalog No.:BCN8434
CAS No.:1177-71-5
- DL-Syringaresinol
Catalog No.:BCN6053
CAS No.:1177-14-6
- LY 255283
Catalog No.:BCC7290
CAS No.:117690-79-6
- Decumbenine C
Catalog No.:BCC8314
CAS No.:117772-89-1
- Desmethyl-YM 298198
Catalog No.:BCC7365
CAS No.:1177767-57-5
- AP-III-a4
Catalog No.:BCC5292
CAS No.:1177827-73-4
- NSC 23766
Catalog No.:BCC1149
CAS No.:1177865-17-6
- 3,3',4',5,6,7,8-heptamethoxyflavone
Catalog No.:BCN8203
CAS No.:1178-24-1
- Enterostatin
Catalog No.:BCC6050
CAS No.:117830-79-2
- Ac-Asp(OtBu)-OH
Catalog No.:BCC2880
CAS No.:117833-18-8
- Loreclezole hydrochloride
Catalog No.:BCC7009
CAS No.:117857-45-1
- L-CCG-l
Catalog No.:BCC6609
CAS No.:117857-93-9
- L-CCG-lll
Catalog No.:BCC6608
CAS No.:117857-95-1
- Fmoc-Thr(Bzl)-OH
Catalog No.:BCC3550
CAS No.:117872-75-0
- 7,4'-Dihydroxyhomoisoflavanone
Catalog No.:BCN3582
CAS No.:1178893-64-5
Development, characterization and solubility study of solid dispersions of azithromycin dihydrate by solvent evaporation method.[Pubmed:22247849]
J Adv Pharm Technol Res. 2010 Apr;1(2):221-8.
Azithromycin Dihydrate (Poorly water soluble drug), when prepared as solid dispersion showed improved solubility and dissolution. So the main purpose of this investigation was to increase the solubility and dissolution rate of Azithromycin Dihydrate by the preparation of its solid dispersion with urea using solvent evaporation method. Physical mixtures and solid dispersions of Azithromycin Dihydrate were prepared by using urea as water-soluble carrier in various proportions (1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7 by weight), by employing solvent evaporation method. The drug release profile was studied and it was found that the dissolution rate and the dissolution parameters of the drug from the physical mixture as well as solid dispersion were higher than those of the intact drug. FT- IR spectra revealed no chemical incompatibility between drug and urea. Drug-polymer interactions were investigated using differential scanning calorimetry (DSC) and Powder X-Ray Diffraction (PXRD).
Efficacy of azithromycin dihydrate in treatment of cryptosporidiosis in naturally infected dairy calves.[Pubmed:16095179]
J Vet Intern Med. 2005 Jul-Aug;19(4):590-3.
The objective of this study was to evaluate the therapeutic efficacy of azithromycin treatment of cryptosporidiosis in naturally infected calves under field conditions. Fifty Holstein calves with cryptosporidiosis infection were divided into 5 groups: 1 group (10 calves) was unmedicated and served as the control group and was given distilled water only, whereas the other groups (10 animals per group) were medicated orally with azithromycin at the doses of 500 (group 1), 1,000 (group 2), 1,500 (group 3), and 2,000 mg (group 4) PO once daily for 7 days. The animals were examined clinically and fecal samples were collected on the 1st (inclusion day), 7th, 14th, and 21st days of the study. Drug efficacy was assessed by evaluating diarrhea, oocyst shedding, and weight gains from days 1 to 21 (4 assessments). Significant differences were observed in reductions of oocyst shedding (P < .05) and the fecal diarrhea incidence (P < .05) in groups 3 and 4 when compared with groups 1 and 2 and the control group. Weight gain of medicated calves was significantly higher than that of the unmedicated calves throughout the study (P < .05). The drug significantly suppressed oocyst shedding and resulted in significant improvements in clinical signs. Therefore, this suppression may have significant effect on the reduction of environmental contamination by cryptosporidial oocysts. From the economic point view, authors suggest that the most effective dose of azithromycin for the treatment of cryptosporidiosis in calves should be at 1,500 mg/d for 7 days.


