Alda 1ALDH2 activator CAS# 349438-38-6 |
- Gimeracil
Catalog No.:BCC2296
CAS No.:103766-25-2
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- Trilostane
Catalog No.:BCC2302
CAS No.:13647-35-3
- PluriSIn #1 (NSC 14613)
Catalog No.:BCC2305
CAS No.:91396-88-2
- CPI-613
Catalog No.:BCC2287
CAS No.:95809-78-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 349438-38-6 | SDF | Download SDF |
| PubChem ID | 831629 | Appearance | Powder |
| Formula | C15H11Cl2NO3 | M.Wt | 324.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 51 mg/mL (157.33 mM) *"≥" means soluble, but saturation unknown. | ||
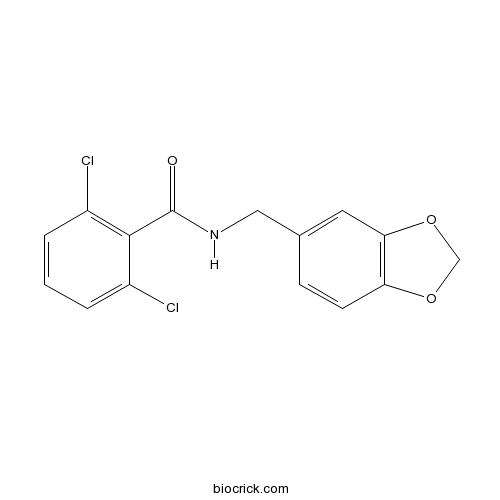
| Chemical Name | N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide | ||
| SMILES | C1OC2=C(O1)C=C(C=C2)CNC(=O)C3=C(C=CC=C3Cl)Cl | ||
| Standard InChIKey | NMKJFZCBCIUYHI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H11Cl2NO3/c16-10-2-1-3-11(17)14(10)15(19)18-7-9-4-5-12-13(6-9)21-8-20-12/h1-6H,7-8H2,(H,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Activator of aldehyde dehydrogenase 2 (ALDH2). Increases activity of wild-type ALDH2*1 and variant ALDH2*2 (by ~2-fold and 11-fold respectively). Capable of partly restoring mutant ALDH2*2 activity; protective against cardiac ischemia. |

Alda 1 Dilution Calculator

Alda 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0849 mL | 15.4245 mL | 30.849 mL | 61.6979 mL | 77.1224 mL |
| 5 mM | 0.617 mL | 3.0849 mL | 6.1698 mL | 12.3396 mL | 15.4245 mL |
| 10 mM | 0.3085 mL | 1.5424 mL | 3.0849 mL | 6.1698 mL | 7.7122 mL |
| 50 mM | 0.0617 mL | 0.3085 mL | 0.617 mL | 1.234 mL | 1.5424 mL |
| 100 mM | 0.0308 mL | 0.1542 mL | 0.3085 mL | 0.617 mL | 0.7712 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
Alda 1 is an activator of aldehyde dehydrogenase 2 (ALDH2).
Alda 1 increases activity of wild-type ALDH2*1 and variant ALDH2*2 (by ~2-fold and 11-fold respectively), Capable of partly restoring mutant ALDH2*2 activity, and protective against cardiac ischemia.
In vitro: In the present study , Alda-1 increased acetaldehyde oxidation by ALDH2*1 and ALDH2*2 approximately 1.5- and 6-fold, respectively, regarding GTN bioactivation and the effects of Alda-1. To similar extent, Alda-1 induced the esterase activities of both enzymes as the coenzyme NAD. NAD pronounced inhibition occurring at > 5mM, its the effect was biphasic. In the presence of 1 mM NAD, Alda-1 induced ALDH2*2-catalyzed ester hydrolysis 73-fold, whereas the NAD-induced activity of ALDH2*1 was inhibited on account of 20-fold increased inhibitory potency of NAD in the presence of the drug. ALDH2*2 displayed 7-fold lower GTN denitrating activity and GTN affinity than ALDH2*1, but the rate of nitric oxide formation was only reduced 2-fold. Soluble guanylate cyclase (sGC) activation was more pronounced than with wild type ALDH2 at saturating GTN. Alda-1 caused slight inhibition of GTN denitration, and in the presence of either variant, GTN-induced sGC activation was increased. The present results imply that established ALDH2 activities stimulated by Alda-1, which improved NAD binding but does not improve the GTN binding affinity of the Asian variant. In addition, an unexpected discrepancy between GTN reductase activity and sGC activation was revealed, which suggested that GTN denitration and bioactivation may display independent pathways of ALDH2-catalyzed GTN biotransformation [1].
In vivo: Alda-1 (a high-throughput screen yielded a small-molecule activator of ALDH2, administered to rats before an ischemic event) reduced infarct size by 60%, most likely through the inhibition of the formation of cytotoxic aldehydes. Thus, pharmacologic enhancement of ALDH2 activity may be benefit to patients with wild-type or mutant ALDH2 who are subjected to cardiac ischemia, such as during coronary bypass surgery [2].
Clinical trial: Clinical study has been conducted.
References:
[1]. Beretta M, Gorren AC, Wenzl MV, Weis R, Russwurm M, Koesling D, Schmidt K, Mayer B. Characterization of the East Asian variant of aldehyde dehydrogenase-2: bioactivation of nitroglycerin and effects of Alda-1. J Biol Chem. 2010 Jan 8;285(2):943-52.
[2]. Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008 Sep 12;321(5895):1493-5.
- S-Adenosyl-L-Methionine iodide salt
Catalog No.:BCN2232
CAS No.:3493-13-8
- Cryptomeridiol 11-rhamnoside
Catalog No.:BCN4648
CAS No.:349112-30-7
- JX 401
Catalog No.:BCC7443
CAS No.:349087-34-9
- GSK 137647
Catalog No.:BCC8045
CAS No.:349085-82-1
- HC-030031
Catalog No.:BCC4910
CAS No.:349085-38-7
- Vineridine
Catalog No.:BCN5286
CAS No.:3489-06-3
- UK 383367
Catalog No.:BCC2308
CAS No.:348622-88-8
- Palmatine
Catalog No.:BCN5285
CAS No.:3486-67-7
- Coptisine
Catalog No.:BCN6320
CAS No.:3486-66-6
- Optovin
Catalog No.:BCC6323
CAS No.:348575-88-2
- Methyl 3-methoxyacrylate
Catalog No.:BCN2258
CAS No.:34846-90-7
- 12-Hydroxyabietic acid
Catalog No.:BCN5284
CAS No.:3484-61-5
- Methyl dodonate A acetate
Catalog No.:BCN4647
CAS No.:349487-98-5
- Methyl dodonate A
Catalog No.:BCN4646
CAS No.:349534-70-9
- Dodonolide
Catalog No.:BCN4645
CAS No.:349534-73-2
- 3'',4''-Di-O-acetyl-2'',6''-di-O-p-coumaroylastragalin
Catalog No.:BCN6843
CAS No.:349545-02-4
- Z-Val-OSu
Catalog No.:BCC2733
CAS No.:3496-11-5
- Kushenol F
Catalog No.:BCN3798
CAS No.:34981-24-3
- Kuraridine
Catalog No.:BCN2988
CAS No.:34981-25-4
- Kurarinone
Catalog No.:BCN2985
CAS No.:34981-26-5
- 4alpha,6alpha-Dihydroxyeudesm-11(13)-en-12,8beta-olide
Catalog No.:BCN7431
CAS No.:35001-19-5
- 10beta-Hydroxycadina-4,11(13)-dien-12,8beta-olide
Catalog No.:BCN7435
CAS No.:35001-23-1
- Cucurbitadienol
Catalog No.:BCN3342
CAS No.:35012-08-9
- NHS-Biotin
Catalog No.:BCC3577
CAS No.:35013-72-0
Pretreatment with the ALDH2 agonist Alda-1 reduces intestinal injury induced by ischaemia and reperfusion in mice.[Pubmed:28325855]
Clin Sci (Lond). 2017 Jun 1;131(11):1123-1136.
Many studies demonstrate that activation of aldehyde dehydrogenase 2 (ALDH2) protects against oxidative stress via detoxification of cytotoxic aldehydes, and could attenuate cardiac, cerebral, lung and renal ischaemia-reperfusion (I/R) injuries. However, the effect of ALDH2 in intestinal I/R is unknown. The present study was set up to determine whether an ALDH2 agonist, Alda-1, could alleviate intestinal injury after gut I/R. In a mouse model of intestinal I/R injury, histological grading, proinflammatory cytokines, oxidative stress, cellular apoptosis, chemokine contents, ALDH2 activity, 4-hydroxy-trans-2-nonenal (4-HNE) and malondialdehyde (MDA) were evaluated. The results indicated that I/R treatment conferred elevation in pathological scores, proinflammatory cytokines, oxidative stress, cellular apoptosis and chemokine levels, accompanied by accumulated 4-HNE and MDA. No significant changes in ALDH2 activity were observed after I/R. However, Alda-1 pretreatment significantly decreased these injurious indicators, concomitant with up-regulated ALDH2 activity, and lessened 4-HNE and MDA accumulation. Taken together, our results implicate activation of ALDH2 by Alda-1 in the significant abatement intestinal I/R injury.
Proteomic Analysis of Mitochondria-Enriched Fraction Isolated from the Frontal Cortex and Hippocampus of Apolipoprotein E Knockout Mice Treated with Alda-1, an Activator of Mitochondrial Aldehyde Dehydrogenase (ALDH2).[Pubmed:28218653]
Int J Mol Sci. 2017 Feb 17;18(2). pii: ijms18020435.
The role of different genotypes of apolipoprotein E (apoE) in the etiology of Alzheimer's disease is widely recognized. It has been shown that altered functioning of apoE may promote 4-hydroxynonenal modification of mitochondrial proteins, which may result in mitochondrial dysfunction, aggravation of oxidative stress, and neurodegeneration. Mitochondrial aldehyde dehydrogenase (ALDH2) is an enzyme considered to perform protective function in mitochondria by the detoxification of the end products of lipid peroxidation, such as 4-hydroxynonenal and other reactive aldehydes. The goal of our study was to apply a differential proteomics approach in concert with molecular and morphological techniques to elucidate the changes in the frontal cortex and hippocampus of apolipoprotein E knockout (apoE(-/-)) mice upon treatment with Alda-1-a small molecular weight activator of ALDH2. Despite the lack of significant morphological changes in the brain of apoE(-/-) mice as compared to age-matched wild type animals, the proteomic and molecular approach revealed many changes in the expression of genes and proteins, indicating the impairment of energy metabolism, neuroplasticity, and neurogenesis in brains of apoE(-/-) mice. Importantly, prolonged treatment of apoE(-/-) mice with Alda-1 led to the beneficial changes in the expression of genes and proteins related to neuroplasticity and mitochondrial function. The pattern of alterations implies mitoprotective action of Alda-1, however, the accurate functional consequences of the revealed changes require further research.
In Vivo Post-Cardiac Arrest Myocardial Dysfunction Is Supported by Ca2+/Calmodulin-Dependent Protein Kinase II-Mediated Calcium Long-Term Potentiation and Mitigated by Alda-1, an Agonist of Aldehyde Dehydrogenase Type 2.[Pubmed:27582424]
Circulation. 2016 Sep 27;134(13):961-977.
BACKGROUND: Survival after sudden cardiac arrest is limited by postarrest myocardial dysfunction, but understanding of this phenomenon is constrained by a lack of data from a physiological model of disease. In this study, we established an in vivo model of cardiac arrest and resuscitation, characterized the biology of the associated myocardial dysfunction, and tested novel therapeutic strategies. METHODS: We developed rodent models of in vivo postarrest myocardial dysfunction using extracorporeal membrane oxygenation resuscitation followed by invasive hemodynamics measurement. In postarrest isolated cardiomyocytes, we assessed mechanical load and Ca(2) (+)-induced Ca(2+) release (CICR) simultaneously using the microcarbon fiber technique and observed reduced function and myofilament calcium sensitivity. We used a novel fiberoptic catheter imaging system and a genetically encoded calcium sensor, GCaMP6f, to image CICR in vivo. RESULTS: We found potentiation of CICR in isolated cells from this extracorporeal membrane oxygenation model and in cells isolated from an ischemia/reperfusion Langendorff model perfused with oxygenated blood from an arrested animal but not when reperfused in saline. We established that CICR potentiation begins in vivo. The augmented CICR observed after arrest was mediated by the activation of Ca(2+)/calmodulin-dependent protein kinase II (CaMKII). Increased phosphorylation of CaMKII, phospholamban, and ryanodine receptor 2 was detected in the postarrest period. Exogenous adrenergic activation in vivo recapitulated Ca(2+) potentiation but was associated with lesser CaMKII activation. Because oxidative stress and aldehydic adduct formation were high after arrest, we tested a small-molecule activator of aldehyde dehydrogenase type 2, Alda-1, which reduced oxidative stress, restored calcium and CaMKII homeostasis, and improved cardiac function and postarrest outcome in vivo. CONCLUSIONS: Cardiac arrest and reperfusion lead to CaMKII activation and calcium long-term potentiation, which support cardiomyocyte contractility in the face of impaired postarrest myofilament calcium sensitivity. Alda-1 mitigates these effects, normalizes calcium cycling, and improves outcome.
The aldehyde dehydrogenase, AldA, is essential for L-1,2-propanediol utilization in laboratory-evolved Escherichia coli.[Pubmed:27938862]
Microbiol Res. 2017 Jan;194:47-52.
Most Escherichia coli strains are naturally unable to grow on 1,2-propanediol (PDO) as a sole carbon source. Recently, however, a K-12 descendent E. coli strain was evolved to grow on 1,2-PDO, and it was hypothesized that this evolved ability was dependent on the aldehyde dehydrogenase, AldA, which is highly conserved among members of the family Enterobacteriacea. To test this hypothesis, we first performed computational model simulation, which confirmed the essentiality of the aldA gene for 1,2-PDO utilization by the evolved PDO-degrading E. coli. Next, we deleted the aldA gene from the evolved strain, and this deletion was sufficient to abolish the evolved phenotype. On re-introducing the gene on a plasmid, the evolved phenotype was restored. These findings provide experimental evidence for the computationally predicted role of AldA in 1,2-PDO utilization, and represent a good example of E. coli robustness, demonstrated by the bacterial deployment of a generalist enzyme (here AldA) in multiple pathways to survive carbon starvation and to grow on a non-native substrate when no native carbon source is available.
Characterization of the East Asian variant of aldehyde dehydrogenase-2: bioactivation of nitroglycerin and effects of Alda-1.[Pubmed:19906643]
J Biol Chem. 2010 Jan 8;285(2):943-52.
The East Asian variant of mitochondrial aldehyde dehydrogenase (ALDH2) exhibits significantly reduced dehydrogenase, esterase, and nitroglycerin (GTN) denitrating activities. The small molecule Alda-1 was reported to partly restore low acetaldehyde dehydrogenase activity of this variant. In the present study we compared the wild type enzyme (ALDH2*1) with the Asian variant (ALDH2*2) regarding GTN bioactivation and the effects of Alda-1. Alda-1 increased acetaldehyde oxidation by ALDH2*1 and ALDH2*2 approximately 1.5- and 6-fold, respectively, and stimulated the esterase activities of both enzymes to similar extent as the coenzyme NAD. The effect of NAD was biphasic with pronounced inhibition occurring at > or = 5 mM. In the presence of 1 mM NAD, Alda-1 stimulated ALDH2*2-catalyzed ester hydrolysis 73-fold, whereas the NAD-stimulated activity of ALDH2*1 was inhibited because of 20-fold increased inhibitory potency of NAD in the presence of the drug. Although ALDH2*2 exhibited 7-fold lower GTN denitrating activity and GTN affinity than ALDH2*1, the rate of nitric oxide formation was only reduced 2-fold, and soluble guanylate cyclase (sGC) activation was more pronounced than with wild type ALDH2 at saturating GTN. Alda-1 caused slight inhibition of GTN denitration and did not increase GTN-induced sGC activation in the presence of either variant. The present results indicate that Alda-1 stimulates established ALDH2 activities by improving NAD binding but does not improve the GTN binding affinity of the Asian variant. In addition, our data revealed an unexpected discrepancy between GTN reductase activity and sGC activation, suggesting that GTN denitration and bioactivation may reflect independent pathways of ALDH2-catalyzed GTN biotransformation.
Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart.[Pubmed:18787169]
Science. 2008 Sep 12;321(5895):1493-5.
There is substantial interest in the development of drugs that limit the extent of ischemia-induced cardiac damage caused by myocardial infarction or by certain surgical procedures. Here, using an unbiased proteomic search, we identified mitochondrial aldehyde dehydrogenase 2 (ALDH2) as an enzyme whose activation correlates with reduced ischemic heart damage in rodent models. A high-throughput screen yielded a small-molecule activator of ALDH2 (Alda-1) that, when administered to rats before an ischemic event, reduced infarct size by 60%, most likely through its inhibitory effect on the formation of cytotoxic aldehydes. In vitro, Alda-1 was a particularly effective activator of ALDH2*2, an inactive mutant form of the enzyme that is found in 40% of East Asian populations. Thus, pharmacologic enhancement of ALDH2 activity may be useful for patients with wild-type or mutant ALDH2 who are subjected to cardiac ischemia, such as during coronary bypass surgery.


