680C91Potent and selective tryptophan 2,3-dioxygenase (TDO) inhibitor CAS# 163239-22-3 |
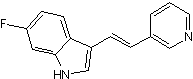
- Edoxaban tosylate monohydrate
Catalog No.:BCC1545
CAS No.:1229194-11-9
- Otamixaban
Catalog No.:BCC1827
CAS No.:193153-04-7
- Betrixaban
Catalog No.:BCC5118
CAS No.:330942-05-7
- Rivaroxaban
Catalog No.:BCC2292
CAS No.:366789-02-8
- Edoxaban
Catalog No.:BCC1543
CAS No.:480449-70-5
- Apixaban
Catalog No.:BCC2295
CAS No.:503612-47-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure
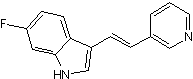
3D structure
| Cas No. | 163239-22-3 | SDF | Download SDF |
| PubChem ID | 10014426 | Appearance | Powder |
| Formula | C15H11FN2 | M.Wt | 238.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in ethanol | ||
| Chemical Name | 6-fluoro-3-[(E)-2-pyridin-3-ylethenyl]-1H-indole | ||
| SMILES | C1=CC(=CN=C1)C=CC2=CNC3=C2C=CC(=C3)F | ||
| Standard InChIKey | YBSDQTBCNYWBMX-ONEGZZNKSA-N | ||
| Standard InChI | InChI=1S/C15H11FN2/c16-13-5-6-14-12(10-18-15(14)8-13)4-3-11-2-1-7-17-9-11/h1-10,18H/b4-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective inhibitor of tryptophan 2,3-dioxygenase (TDO) (Ki = 51 nM). Exhibits no activity against indoleamine 2,3-dioxygenase, monoamine oxidase A and B, 5-HT uptake or 5-HT1A, 1D, 2A and 2C receptors. Produces large increases in brain tryptophan and serotonin in vitro and in vivo in the rat. |

680C91 Dilution Calculator

680C91 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1971 mL | 20.9855 mL | 41.971 mL | 83.9419 mL | 104.9274 mL |
| 5 mM | 0.8394 mL | 4.1971 mL | 8.3942 mL | 16.7884 mL | 20.9855 mL |
| 10 mM | 0.4197 mL | 2.0985 mL | 4.1971 mL | 8.3942 mL | 10.4927 mL |
| 50 mM | 0.0839 mL | 0.4197 mL | 0.8394 mL | 1.6788 mL | 2.0985 mL |
| 100 mM | 0.042 mL | 0.2099 mL | 0.4197 mL | 0.8394 mL | 1.0493 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ezetimibe
Catalog No.:BCN2180
CAS No.:163222-33-1
- Cimifugin 4'-O-beta-D-glucopyranoside
Catalog No.:BCN7853
CAS No.:1632110-81-6
- (-)-[3R,4S]-Chromanol 293B
Catalog No.:BCC7080
CAS No.:163163-24-4
- Chromanol 293B
Catalog No.:BCC7055
CAS No.:163163-23-3
- Cannabisin F
Catalog No.:BCN4696
CAS No.:163136-19-4
- BYK 49187
Catalog No.:BCC2450
CAS No.:163120-31-8
- N-Benzylmaleimide
Catalog No.:BCC9095
CAS No.:1631-26-1
- Albatrelin G
Catalog No.:BCN7596
CAS No.:1630970-05-6
- 17-Hydroxy-18-dehydroneogrifolin
Catalog No.:BCN7633
CAS No.:1630936-42-3
- Huperzine C
Catalog No.:BCN2489
CAS No.:163089-71-2
- (1S,2S)-1-Amino-2-Indanol
Catalog No.:BCC8386
CAS No.:163061-74-3
- (1R,2R)-1-Amino-2-indanol
Catalog No.:BCC8380
CAS No.:163061-73-2
- Clevudine
Catalog No.:BCC4770
CAS No.:163252-36-6
- Sitafloxacin Hydrate
Catalog No.:BCC4959
CAS No.:163253-35-8
- Bethoxazin
Catalog No.:BCC5471
CAS No.:163269-30-5
- FIIN-2
Catalog No.:BCC3974
CAS No.:1633044-56-0
- Stachybotrylactam
Catalog No.:BCN6967
CAS No.:163391-76-2
- 2',4'-Di-O-(E-p-coumaroyl)afzelin
Catalog No.:BCN6512
CAS No.:163434-73-9
- Fmoc-Met(O2)-OH
Catalog No.:BCC3531
CAS No.:163437-14-7
- Flufenamic acid
Catalog No.:BCC9162
CAS No.:530-78-9
- Triptoquinonide
Catalog No.:BCN1724
CAS No.:163513-81-3
- Vilazodone Hydrochloride
Catalog No.:BCC2041
CAS No.:163521-08-2
- Vilazodone
Catalog No.:BCC2040
CAS No.:163521-12-8
- Kadsulignan N
Catalog No.:BCN3631
CAS No.:163564-58-7
Differential expression and regulation of Tdo2 during mouse decidualization.[Pubmed:24190896]
J Endocrinol. 2013 Dec 2;220(1):73-83.
Tryptophan 2,3-dioxygenase (Tdo2) is a rate-limiting enzyme which directs the conversion of tryptophan to kynurenine. The aim of this study was to examine the expression and regulation of Tdo2 in mouse uterus during decidualization. Tdo2 mRNA was mainly expressed in the decidua on days 6-8 of pregnancy. By real-time PCR, a high level of Tdo2 expression was observed in the uteri from days 6 to 8 of pregnancy, although Tdo2 expression was observed on days 1-8. Simultaneously, Tdo2 mRNA was also detected under in vivo and in vitro artificial decidualization. Estrogen, progesterone, and 8-bromoadenosine-cAMP could induce the expression of Tdo2 in the ovariectomized mouse uterus and uterine stromal cells. Tdo2 could regulate cell proliferation and stimulate the expression of decidual marker Dtprp in the uterine stromal cells and decidual cells. Overexpression of Tdo2 could upregulate the expression of Ahr, Cox2, and Vegf genes in uterine stromal cells, while Tdo2 inhibitor 680C91 could downregulate the expression of Cox2 and Vegf genes in uterine decidual cells. These data indicate that Tdo2 may play an important role during mouse decidualization and be regulated by estrogen, progesterone, and cAMP.
The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism in the rat.[Pubmed:7539265]
Biochem Pharmacol. 1995 May 17;49(10):1435-42.
The effects of a novel inhibitor 680C91 ((E)-6-fluoro-3-[2-(3- pyridyl)vinyl]-1H-indole) of the key enzyme of tryptophan catabolism tryptophan 2,3-dioxygenase (TDO) (EC 1.13.11.11), were examined on tryptophan catabolism in vitro and in vivo and on brain levels of tryptophan, serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA). 680C91 was a potent (Ki = 51 nM) and selective TDO inhibitor with no inhibitory activity against indoleamine 2,3-dioxygenase (EC 1.13.11.17), monoamine oxidase A and B, 5-HT uptake and 5-HT1A,1D,2A and 2C receptors at a concentration of 10 microM. 680C91 had no effect on the binding of tryptophan to serum albumin in plasma and inhibited TDO competitively with respect to its substrate tryptophan. 680C91 inhibited the catabolism of tryptophan by rat liver cells and rat liver perfused in situ. The catabolism of L-[ring-2-14C]-tryptophan and a load dose of tryptophan (100 mg/kg) in vivo were inhibited by prior administration of 680C91. Administration of 680C91 alone produced marked increases in brain tryptophan, 5-HT and 5-HIAA. A load dose of tryptophan (100 mg/kg), producing increases in brain tryptophan 4-fold greater than that seen with 680C91, did not increase brain 5-HT and 5-HIAA to levels greater than those seen with 680C91 and produced a shorter-lasting increase in these parameters. These data therefore demonstrate the importance of TDO as a regulator of whole-body tryptophan catabolism and brain levels of tryptophan and 5-HT and suggest that a greater antidepressant efficacy might be achieved with inhibitors of TDO than tryptophan administration alone.
The effects of an inhibitor of tryptophan 2,3-dioxygenase and a combined inhibitor of tryptophan 2,3-dioxygenase and 5-HT reuptake in the rat.[Pubmed:7617147]
Neuropharmacology. 1995 Feb;34(2):217-27.
The effects of a novel inhibitor 680C91 ((E)-6-fluoro-3-[2-(3-pyridyl)vinyl]-1H-indole) of the key enzyme of tryptophan catabolism tryptophan 2,3-dioxygenase (TDO), and a novel inhibitor 709W92 ((E)-6-fluoro-3-[2-(4-pyridyl)vinyl]-1H-indole), of both TDO and 5-hydroxytryptamine (5-HT) reuptake, were examined on tryptophan catabolism, cerebrospinal fluid (CSF) concentrations of tryptophan and 5-HT and serotonergic-mediated physiology and behaviour in the rat. The catabolism of L-[ring-2-14C]tryptophan in vivo was completely inhibited by prior administration of 709W92. 709W92, but not 680C91, potentiated head-twitch produced by 5-hydroxytryptophan, prevented head-twitch and whole brain 5-HT depletion produced by p-chloroamphetamine and rapidly decreased dorsal raphe firing. Both 709W92 and 680C91 elevated CSF tryptophan by up to 260% of basal concentration. A maximally effective dose of 680C91 elevated a global measure of brain extracellular 5-HT (CSF 5-HT) to concentrations similar to those seen maximally after exogenous tryptophan administration (approx 170% of basal). Maximally effective doses of 709W92 increased CSF 5-HT to concentrations comparable to those seen after tryptophan and 5-HT reuptake inhibitor coadministration (approx 900% of basal) and to concentrations greater than those achieved maximally with serotonergically active antidepressant monotherapy (approx 500% of basal). 709W92 did not elevate CSF 5-HT to concentrations associated with the serotonin syndrome (approx 3000% of basal). The combined TDO inhibitor/5-HT reuptake inhibitor, 709W92, showed anxiolytic activity in the rat-pup vocalization model of anxiety. These results show that 709W92 (a novel inhibitor of both TDO and 5-HT reuptake), can produce an elevation of CSF 5-HT similar to that achieved with a serotonin reuptake inhibitor/tryptophan combination therapy but with a more sustained timecourse; such compounds may therefore have superior antidepressant efficacy in the clinic.
An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor.[Pubmed:21976023]
Nature. 2011 Oct 5;478(7368):197-203.
Activation of the aryl hydrocarbon receptor (AHR) by environmental xenobiotic toxic chemicals, for instance 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin), has been implicated in a variety of cellular processes such as embryogenesis, transformation, tumorigenesis and inflammation. But the identity of an endogenous ligand activating the AHR under physiological conditions in the absence of environmental toxic chemicals is still unknown. Here we identify the tryptophan (Trp) catabolite kynurenine (Kyn) as an endogenous ligand of the human AHR that is constitutively generated by human tumour cells via tryptophan-2,3-dioxygenase (TDO), a liver- and neuron-derived Trp-degrading enzyme not yet implicated in cancer biology. TDO-derived Kyn suppresses antitumour immune responses and promotes tumour-cell survival and motility through the AHR in an autocrine/paracrine fashion. The TDO-AHR pathway is active in human brain tumours and is associated with malignant progression and poor survival. Because Kyn is produced during cancer progression and inflammation in the local microenvironment in amounts sufficient for activating the human AHR, these results provide evidence for a previously unidentified pathophysiological function of the AHR with profound implications for cancer and immune biology.


