3,4-Dihydroxyphenylacetic AcidCAS# 102-32-9 |
Quality Control & MSDS
Number of papers citing our products

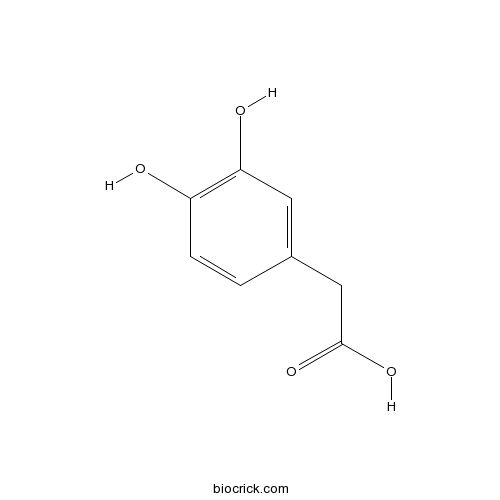
Chemical structure

3D structure
| Cas No. | 102-32-9 | SDF | Download SDF |
| PubChem ID | 547 | Appearance | Powder |
| Formula | C8H8O4 | M.Wt | 168 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(3,4-dihydroxyphenyl)acetic acid | ||
| SMILES | C1=CC(=C(C=C1CC(=O)O)O)O | ||
| Standard InChIKey | CFFZDZCDUFSOFZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8O4/c9-6-2-1-5(3-7(6)10)4-8(11)12/h1-3,9-10H,4H2,(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3,4-Dihydroxybenzoic acid acts as a potential ALDH inducer to prevent the alcohol-induced abnormal reaction. It has antioxidant activity, it exhibited both DPPH radical scavenging and superoxide dismutase-like activities. |
| In vitro | 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides.[Pubmed: 28460970 ]Food Res Int. 2016 Nov;89(Pt 1):716-723.Since dietary flavonoid glycosides, including quercetin 4'-glucoside from onion, are poorly absorbed from the gastrointestinal tract, they are converted into smaller phenolic acids, which can be absorbed into the circulation. The purpose of this study was to compare the effects of the major phenolic acid catabolites of quercetin 4'-glucoside, including 3,4-Dihydroxyphenylacetic acid (DOPAC), 3-hydroxyphenylacetic acid, 3,4-dihydroxybenzoic acid (protocatechuic acid) and hippuric acid, on the antioxidant activity and phase II cytoprotective enzyme induction in vitro. 3,4-Dihydroxyphenylacetic acid is a potential aldehyde dehydrogenase inducer in murine hepatoma Hepa1c1c7 cells.[Pubmed: 28828965 ]Biosci Biotechnol Biochem. 2017 Oct;81(10):1978-1983.3,4-Dihydroxyphenylacetic acid (DOPAC) is one of the major colonic microflora-produced catabolites of quercetin glycosides, such as quercetin 4'-glucoside derived from onion. |
| Structure Identification | Acta Crystallographica Section E Structure Reports Online, 2001, 57(8).3,4-Dihydroxyphenylacetic acid[Reference: WebLink]
|

3,4-Dihydroxyphenylacetic Acid Dilution Calculator

3,4-Dihydroxyphenylacetic Acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.9524 mL | 29.7619 mL | 59.5238 mL | 119.0476 mL | 148.8095 mL |
| 5 mM | 1.1905 mL | 5.9524 mL | 11.9048 mL | 23.8095 mL | 29.7619 mL |
| 10 mM | 0.5952 mL | 2.9762 mL | 5.9524 mL | 11.9048 mL | 14.881 mL |
| 50 mM | 0.119 mL | 0.5952 mL | 1.1905 mL | 2.381 mL | 2.9762 mL |
| 100 mM | 0.0595 mL | 0.2976 mL | 0.5952 mL | 1.1905 mL | 1.4881 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Acetoacetanilide
Catalog No.:BCC8803
CAS No.:102-01-2
- GW791343 dihydrochloride
Catalog No.:BCC1613
CAS No.:1019779-04-4
- Zardaverine
Catalog No.:BCC2069
CAS No.:101975-10-4
- Octacosyl (E)-ferulate
Catalog No.:BCN5834
CAS No.:101959-37-9
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Regorafenib monohydrate
Catalog No.:BCC1884
CAS No.:1019206-88-2
- Dabigatran etexilate benzenesulfonate
Catalog No.:BCC8925
CAS No.:1019206-65-5
- sodium 4-pentynoate
Catalog No.:BCC1958
CAS No.:101917-30-0
- LX-4211
Catalog No.:BCC1714
CAS No.:1018899-04-1
- 7-Z-Trifostigmanoside I
Catalog No.:BCN7869
CAS No.:1018898-17-3
- Diclazuril
Catalog No.:BCC8937
CAS No.:101831-37-2
- Phenyethyl 3-methylcaffeate
Catalog No.:BCN8457
CAS No.:71835-85-3
- Sulfaclozine
Catalog No.:BCC9155
CAS No.:102-65-8
- 20,24-Epoxy-24-methoxy-23(24-25)abeo-dammaran-3-one
Catalog No.:BCN1639
CAS No.:1020074-97-8
- SGI-1027
Catalog No.:BCC4588
CAS No.:1020149-73-8
- DCC-2036 (Rebastinib)
Catalog No.:BCC4390
CAS No.:1020172-07-9
- (R)-4-Benzyl-2-oxazolidinone
Catalog No.:BCC8395
CAS No.:102029-44-7
- PF-04457845
Catalog No.:BCC1851
CAS No.:1020315-31-4
- Protosappanin A
Catalog No.:BCN7259
CAS No.:102036-28-2
- Protosappanin B
Catalog No.:BCN2281
CAS No.:102036-29-3
- Tubeimoside I
Catalog No.:BCN1089
CAS No.:102040-03-9
- 3-Deazaneplanocin,DZNep
Catalog No.:BCC1129
CAS No.:102052-95-9
- Arctinol B
Catalog No.:BCN5835
CAS No.:102054-39-7
Pharmacokinetics and pharmacodynamics of a single dose Nilotinib in individuals with Parkinson's disease.[Pubmed:30906562]
Pharmacol Res Perspect. 2019 Mar 12;7(2):e00470.
Nilotinib is a broad-based tyrosine kinase inhibitor with the highest affinity to inhibit Abelson (c-Abl) and discoidin domain receptors (DDR1/2). Preclinical evidence indicates that Nilotinib reduces the level of brain alpha-synuclein and attenuates inflammation in models of Parkinson's disease (PD). We previously showed that Nilotinib penetrates the blood-brain barrier (BBB) and potentially improves clinical outcomes in individuals with PD and dementia with Lewy bodies (DLB). We performed a physiologically based population pharmacokinetic/pharmacodynamic (popPK/PD) study to determine the effects of Nilotinib in a cohort of 75 PD participants. Participants were randomized (1:1:1:1:1) into five groups (n = 15) and received open-label random single dose (RSD) 150:200:300:400 mg Nilotinib vs placebo. Plasma and cerebrospinal fluid (CSF) were collected at 1, 2, 3, and 4 hours after Nilotinib administration. The results show that Nilotinib enters the brain in a dose-independent manner and 200 mg Nilotinib increases the level of 3,4-Dihydroxyphenylacetic Acid (DOPAC) and homovanillic acid (HVA), suggesting alteration to dopamine metabolism. Nilotinib significantly reduces plasma total alpha-synuclein and appears to reduce CSF oligomeric: total alpha-synuclein ratio. Furthermore, Nilotinib significantly increases the CSF level of triggering receptors on myeloid cells (TREM)-2, suggesting an anti-inflammatory effect. Taken together, 200 mg Nilotinib appears to be an optimal single dose that concurrently reduces inflammation and engages surrogate disease biomarkers, including dopamine metabolism and alpha-synuclein.
Catechol-based inhibitors of bacterial urease.[Pubmed:30850166]
Bioorg Med Chem Lett. 2019 May 1;29(9):1085-1089.
Targeted covalent inhibitors of urease were developed on the basis of the catechol structure. Forty amide and ester derivatives of 3,4-Dihydroxyphenylacetic Acid, caffeic acid, ferulic acid and gallic acid were obtained and screened against Sporosarcinia pasteurii urease. The most active compound, namely propargyl ester of 3,4-Dihydroxyphenylacetic Acid exhibited IC50=518nM andkinact/Ki=1379M(-1)s(-1). Inhibitory activity of this compound was better and toxicity lower than those obtained for the starting compound - catechol. The molecular modelling studies revealed a mode of binding consistent with structure-activity relationships.
[Neurotransmitter disturbances in some parts of the rat brain and their correction under chronic and intermittent alcohol intoxication].[Pubmed:30816093]
Biomed Khim. 2019 Jan;65(1):21-27.
The pool of key neuromediators and some neurotransmitter amino acids in cerebellum, hypothalamus and midbrain of rats exposed to chronic and different variants of interrupted alcohol intoxication was investigated. The most pronounced changes were recorded in midbrain. Chronic alcohol intoxication caused an increase in the concentrations of tyrosine, dopamine, 3,4-Dihydroxyphenylacetic Acid (DOPAC), noradrenaline, tryptophan, serotonin, GABA and aspartate in this part of the rat brain. Interrupted alcohol intoxication with 4 days interval is accompanied by an increase in the content of tyrosine, and noradrenaline. Interrupted alcohol intoxication with 1 day interval leaded to an increase in the concentrations of tyrosine, DOPAC, noradrenalin, tryptophan, GABA, glycine and aspartate. The amino acids composition "Titacin" had a pronounced normalizing effect in the midbrain under interrupted alcohol intoxication with 1 day interval.
Anxiolytic effects of theaflavins via dopaminergic activation in the frontal cortex.[Pubmed:30806570]
Biosci Biotechnol Biochem. 2019 Feb 26:1-6.
Epidemiological investigations have reported that the habit of drinking tea reduces the risk of developing a mental disorder, including anxiety disorder and depression. Theaflavins, black tea polyphenols, show antibacterial and anti-oxidative effects, but their effects on brain function, especially mental condition, have not been elucidated. The present study demonstrated that theaflavins increased dopamine (DA) turnover in the frontal cortex and showed an anxiolytic effect in mice. Theaflavin consumption increased the time spent by mice in the open arms of an elevated plus maze test. Theaflavin administration increased the levels of 3,4-Dihydroxyphenylacetic Acid (DOPAC) and the ratios of DOPAC/DA and (DOPAC+homovanillic acids)/DA indicating DA turnover, in the frontal cortex. These results suggest that the consumption of theaflavins induced anxiolytic effects via activation of the dopaminergic system in the frontal cortex, which support the findings of previous epidemiological studies. Theaflavins in black tea may be helpful to reduce anxiety in daily life. (150/150 words).
Identification and characterization of cytochrome P450 1232A24 and 1232F1 from Arthrobacter sp. and their role in the metabolic pathway of papaverine.[Pubmed:30759214]
J Biochem. 2019 Feb 13. pii: 5318338.
Cytochrome P450 monooxygenases (P450s) play crucial roles in the cell metabolism and provide an unsurpassed diversity of catalyzed reactions. Here, we report the identification and biochemical characterization of two P450s from Arthrobacter sp., a gram-positive organism known to degrade the opium alkaloid papaverine. Combining phylogenetic and genomic analysis suggested physiological roles for P450s in metabolism, and revealed potential gene clusters with redox partners facilitating the reconstitution of the P450 activities in vitro. CYP1232F1 catalyzes the para demethylation of 3,4-dimethoxyphenylacetic acid to homovanillic acid while CYP1232A24 continues demethylation to 3,4-Dihydroxyphenylacetic Acid. Interestingly, the latter enzyme is also able to perform both demethylation steps with preference for the meta position. The crystal structure of CYP1232A24, which shares only 29% identity to previous published structures of P450s helped to rationalize the preferred demethylation specificity for the meta position and also the broader substrate specificity profile. In addition to the detailed characterization of the two P450s using their physiological redox partners, we report the construction of a highly-active whole-cell E. coli biocatalyst expressing CYP1232A24, which formed up to 1.77 g l-1 3,4-Dihydroxyphenylacetic Acid. Our results revealed the P450s' role in the metabolic pathway of papaverine enabling further investigation and application of these biocatalysts.
Some New Findings Regarding the Antiadhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Bacteria.[Pubmed:30746933]
J Agric Food Chem. 2019 Feb 27;67(8):2166-2174.
Findings concerning the antiadhesive activity of cranberry phenolic compounds and their microbial-derived metabolites against Gram-negative ( Escherichia coli ATCC 53503 and DSM 10791) and Gram-positive ( Enterococcus faecalis 04-1) bacteria in T24 cells are reported. A-Type procyanidins (A2 and cinnamtannin B-1) exhibited antiadhesive activity (at concentrations >/=250 muM), a feature that was not observed for B-type procyanidins (B2). The metabolites hippuric acid and alpha-hydroxyhippuric acid also showed effective results at concentrations >/=250 muM. With regard to conjugated metabolites, sulfation seemed to increase the antiadhesive activity of cranberry-derived metabolites as 3-(3,4-dihydroxyphenyl)propionic acid 3- O-sulfate presented active results, unlike its corresponding nonsulfated form. In contrast, methylation decreased antiadhesive activity as 3,4-Dihydroxyphenylacetic Acid was found to be active but not its corresponding methylated form (4-hydroxy-3-methoxyphenylacetic acid). As a whole, this work sustains the antiadhesive activity of cranberry-derived metabolites as one of the mechanisms involved in the beneficial effects of cranberries against urinary tract infections.
Pilose Antler Extracts (PAEs) Protect against Neurodegeneration in 6-OHDA-Induced Parkinson's Disease Rat Models.[Pubmed:30728849]
Evid Based Complement Alternat Med. 2019 Jan 8;2019:7276407.
Parkinson's disease (PD) is one of the most common neurodegenerative diseases worldwide. Although dopamine replacement therapy mitigates motor dysfunction in PD patients, there are no therapeutics that are currently available to reverse neuronal cell death in the substantia nigra pars compacta (SNc), which is the main region for dopamine loss in PD patients. The protein concentration of the Pilose antler extracts (PAEs) was estimated using the Bradford Protein Assay Kit. Hematoxylin and eosin (HE) staining was used to evaluate the protective effect of PAEs on 6-OHDA induced cell death in PD model rats. Immunohistochemistry (IHC) was used to detect the tyrosine hydroxylase (TH) positive neuronal cell in SNc. HPLC-MS was used to detect dopamine (DA), 3,4-Dihydroxyphenylacetic Acid (DOPAC), homovanillic acid (HVA), 5-hydroxytryptamine (5-HT), 5-hydroxyindoleacetic acid (5-HIAA), and glutamate (Glu) levels in the striatum and cerebrospinal fluid (CSF). The amino acid level in the striatum and CSF was measured by HPLC-FLD. Protein expression of growth associated protein-43 (GAP-43) and neurofilament heavy polypeptide (NF-H) was measured using western blotting. The components of PAEs through blood vessels were detected by HPLC/MS/MS. In this study, PAEs with proteins ranging from 10 kDa to 250 kDa molecular weight was administered to 6-OHDA-induced PD rats. We found that PAEs inhibited 6-OHDA-induced neuronal cell death and TH-positive neuronal loss in SNc. PAEs administration also increased the levels of DA, DOPAC, and 5-HT, in addition to DOPAC/DA and HVA/DA indexes in the CSF and Striatum of 6-OHDA induced rats. Conversely, PAEs decreased the levels of Glu and GABA. Treatment with PAEs and Madopar increased GAP-43 and NF-H expression in the SNc and striatum. Proteomic analysis using LC/MS/MS indicated that 11 components of PAEs may have neuropharmacological effects. These results demonstrate that PAEs protects against 6-OHDA induced toxic effects in the PD rat models. Intragastric administration of PAEs may be a novel therapeutic strategy for neurodegenerative disorders like PD.
The neuroactive potential of the human gut microbiota in quality of life and depression.[Pubmed:30718848]
Nat Microbiol. 2019 Apr;4(4):623-632.
The relationship between gut microbial metabolism and mental health is one of the most intriguing and controversial topics in microbiome research. Bidirectional microbiota-gut-brain communication has mostly been explored in animal models, with human research lagging behind. Large-scale metagenomics studies could facilitate the translational process, but their interpretation is hampered by a lack of dedicated reference databases and tools to study the microbial neuroactive potential. Surveying a large microbiome population cohort (Flemish Gut Flora Project, n = 1,054) with validation in independent data sets (ntotal = 1,070), we studied how microbiome features correlate with host quality of life and depression. Butyrate-producing Faecalibacterium and Coprococcus bacteria were consistently associated with higher quality of life indicators. Together with Dialister, Coprococcus spp. were also depleted in depression, even after correcting for the confounding effects of antidepressants. Using a module-based analytical framework, we assembled a catalogue of neuroactive potential of sequenced gut prokaryotes. Gut-brain module analysis of faecal metagenomes identified the microbial synthesis potential of the dopamine metabolite 3,4-Dihydroxyphenylacetic Acid as correlating positively with mental quality of life and indicated a potential role of microbial gamma-aminobutyric acid production in depression. Our results provide population-scale evidence for microbiome links to mental health, while emphasizing confounder importance.
Edible nuts deliver polyphenols and their transformation products to the large intestine: An in vitro fermentation model combining targeted/untargeted metabolomics.[Pubmed:30717008]
Food Res Int. 2019 Feb;116:786-794.
The fate of polyphenols from edible tree nuts was investigated using a simulated in vitro intestinal fermentation system. The digested food matrix was fermented for 48h and the changes in the phenolic profiles were evaluated by both untargeted UHPLC-QTOF and targeted UHPLC-Orbitrap mass spectrometry. The untargeted metabolomics approach allowed us to monitor the comprehensive changes in phenolic profiles from 0 up to 48h of in vitro fermentation. Multivariate statistics (i.e., orthogonal projection to latent structures discriminant analysis) applied to this untargeted data allowed us to identify the most discriminating phenolic metabolites and to further understand the colonic transformation pathways involved. In particular, 13 putatively identified compounds derived from flavonoids, lignans and phenolic acids were found to have the highest discrimination potential. Six phenolic metabolites were then quantified by means of targeted metabolomics (using a UHPLC-Orbitrap). These metabolites included 3,4-Dihydroxyphenylacetic Acid, 4-hydroxybenzoic acid, hippuric acid, caffeic acid, protocatechuic acid and protocatechuic aldehyde. Using the targeted data, a clear matrix effect was observed over time, with an increase of some phenolic metabolites moving from 8 to 48h of in vitro fermentation. Based on these data, catabolic pathways for colonic microbial degradation of flavonoids, hydroxycinnamic acids, tyrosols and lignans are proposed. Our findings show that edible tree nuts deliver polyphenols to the colon, where several microbial transformations occur that lead to smaller phenolic metabolites being observed. Furthermore, we found that the combined use of targeted and untargeted metabolomics can be particularly effective for investigating the fate of polyphenols in the large intestine.
Brain Microdialysate Monoamines in Relation to Circadian Rhythms, Sleep, and Sleep Deprivation - a Systematic Review, Network Meta-analysis, and New Primary Data.[Pubmed:30671123]
J Circadian Rhythms. 2019 Jan 14;17:1.
Disruption of the monoaminergic system, e.g. by sleep deprivation (SD), seems to promote certain diseases. Assessment of monoamine levels over the circadian cycle, during different sleep stages and during SD is instrumental to understand the molecular dynamics during and after SD. To provide a complete overview of all available evidence, we performed a systematic review. A comprehensive search was performed for microdialysis and certain monoamines (dopamine, serotonin, noradrenaline, adrenaline), certain monoamine metabolites (3,4-Dihydroxyphenylacetic Acid (DOPAC), 5-hydroxyindoleacetic acid (5-HIAA)) and a precursor (5-hydroxytryptophan (5-HTP)) in PubMed and EMBASE. After screening of the search results by two independent reviewers, 94 publications were included. All results were tabulated and described qualitatively. Network-meta analyses (NMAs) were performed to compare noradrenaline and serotonin concentrations between sleep stages. We further present experimental monoamine data from the medial prefrontal cortical (mPFC). Monoamine levels varied with brain region and circadian cycle. During sleep, monoamine levels generally decreased compared to wake. These qualitative observations were supported by the NMAs: noradrenaline and serotonin levels decreased from wakefulness to slow wave sleep and decreased further during Rapid Eye Movement sleep. In contrast, monoamine levels generally increased during SD, and sometimes remained high even during subsequent recovery. Decreases during or after SD were only reported for serotonin. In our experiment, SD did not affect any of the mPFC monoamine levels. Concluding, monoamine levels vary over the light-dark cycle and between sleep stages. SD modifies the patterns, with effects sometimes lasting beyond the SD period.
Kisspeptin Stimulation of Prolactin Secretion Requires Kiss1 Receptor but Not in Tuberoinfundibular Dopaminergic Neurons.[Pubmed:30668693]
Endocrinology. 2019 Mar 1;160(3):522-533.
Kisspeptin has been shown to stimulate prolactin secretion. We investigated whether kisspeptin acts through the Kiss1 receptor (Kiss1r) to regulate dopamine and prolactin. Initially, we evaluated prolactin response in a Kiss1r-deficient mouse line, in which Kiss1r had been knocked into GnRH neurons (Kiss1r-/-R). Intracerebroventricular kisspeptin-10 (Kp-10) increased prolactin release in wild-type but not in Kiss1r-/-R female mice. In ovariectomized, estradiol-treated rats, the Kiss1r antagonist kisspeptin-234 abolished the Kp-10-induced increase in prolactin release but failed to prevent the concomitant reduction in the activity of tuberoinfundibular dopaminergic (TIDA) neurons, as determined by the 3,4-Dihydroxyphenylacetic Acid/dopamine ratio in the median eminence. Using whole-cell patch clamp recordings in juvenile male rats, we found no direct effect of Kp-10 on the electrical activity of TIDA neurons. In addition, dual-label in situ hybridization in the hypothalamus of female rats showed that Kiss1r is expressed in the periventricular nucleus of the hypothalamus (Pe) and arcuate nucleus of the hypothalamus (ARC) but not in tyrosine hydroxylase (Th)-expressing neurons. Kisspeptin also has affinity for the neuropeptide FF receptor 1 (Npffr1), which was expressed in the majority of Pe dopaminergic neurons but only in a low proportion of TIDA neurons in the ARC. Our findings demonstrate that Kiss1r is necessary to the effect of kisspeptin on prolactin secretion, although TIDA neurons lack Kiss1r and are electrically unresponsive to kisspeptin. Thus, kisspeptin is likely to stimulate prolactin secretion via Kiss1r in nondopaminergic neurons, whereas the colocalization of Npffr1 and Th suggests that Pe dopaminergic neurons may play a role in the kisspeptin-induced inhibition of dopamine release.
Sensitive determination of monoamine neurotransmitters, their main metabolites and precursor amino acids in different mouse brain components by liquid chromatography-electrospray tandem mass spectrometry after selective sample clean-up.[Pubmed:30597586]
Biomed Chromatogr. 2019 Apr;33(4):e4479.
For the assessment of diets and supplements formulated for the treatment of phenylketonuria, a highly sensitive and selective method was developed and validated for the quantification of dopamine (DA), serotonin (5-HT), 3,4-Dihydroxyphenylacetic Acid (DOPAC), 5-hydroxyindoleacetic acid (5-HIAA), phenylalanine, tyrosine and tryptophan in mouse cerebellum, brain stem, hypothalamus, parietal cortex, anterior piriform cortex and bulbus olfactorius. Samples were extracted by deproteinization with acetonitrile, and the extracts were cleaned up by strong anion exchange and weak cation exchange applied sequentially. The substances were detected by rapid liquid chromatography tandem mass spectrometry. Matrix components were largely removed by the clean-up, resulting in low matrix effects. The lower limits of quantification for an extracted tissue mass of 100 mg were 0.3, 0.3, 0.2 and 2 ng/g for DA, 5-HT, 5-HIAA and DOPAC, respectively. The mean true extraction recoveries were 80-102%. The relative intra-laboratory reproducibility standard deviations were generally <11% at concentrations of 20-1000 ng/g for DA, 5-HT, 5-HIAA and DOPAC and 7% at concentrations of 5-50 mug/g for the amino acids. This method was successfully used in a phenylketonuria mice study including nearly 300 brain tissue samples and for small sample masses (for example, 2 mg of bulbus olfactorius).
Analysis of Catecholamine and Their Metabolites in Mice Brain by Liquid Chromatography-Mass Spectrometry Using Sulfonated Mixed-mode Copolymer Column.[Pubmed:30584183]
Anal Sci. 2019 Apr 10;35(4):433-439.
In this study, a simultaneous assay for catecholamines and their metabolites in the brain was established using liquid chromatography-mass spectrometry (LC-MS). To achieve complete separation, a cation-exchange/reversed-phase mixed-mode copolymer resin column containing 0.81 wt% sulfo groups was used for the simultaneous LC-MS assay. The analyzed catecholamines were dopamine (DA), norepinephrine (NE), and epinephrine (E), while the metabolites lacking amino groups were 3,4-Dihydroxyphenylacetic Acid (DOPAC), homovanillic acid (HVA), and 3-methoxy-4-hydroxyphenylglycol (MHPG). The metabolites were separated and detected using LC-MS, on columns with and without sulfo groups. However, we could not achieve adequate separation of catecholamines on both columns using a gradient elution of 0 - 50 (v/v)% methanol containing 0.1 (v/v)% formic acid (FA). When volatile ion-pairing reagents were added to the mobile phase, they improved the retention and detection of catecholamines on the sulfonated mixed-mode column. Under optimized elution conditions, which involved a linear gradient elution of water containing 0.1 (v/v)% FA to 50 (v/v)% acetonitrile in 50 mM ammonium formate at 40 degrees C and a 0.20 mL/min rate, all six target molecules were simultaneously detected within 25 min, when using negative mode LC-MS on a sulfonated mixed-mode column. The limits of detection (LODs) for DA, NE, E, DOPCA, HVA, and MHPG were determined to be 20.7, 12.6, 74.6, 1110, 18.7, and 3196 nM, respectively. Moreover, the established LC-MS assay allowed the detection of endogenous DA, NE, and HVA, in normal mouse brain samples at concentrations higher than 20, 9, and 4 pmol/mg, respectively.


