S0859NBC inhibitor,potent and selective CAS# 1019331-10-2 |
- Sodium Channel inhibitor 1
Catalog No.:BCC1959
CAS No.:1198117-23-5
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Riluzole
Catalog No.:BCC3849
CAS No.:1744-22-5
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
- Dibucaine (Cinchocaine) HCl
Catalog No.:BCC3760
CAS No.:61-12-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1019331-10-2 | SDF | Download SDF |
| PubChem ID | 70675849 | Appearance | Powder |
| Formula | C29H24ClN3O3S | M.Wt | 530.04 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (188.67 mM) *"≥" means soluble, but saturation unknown. | ||
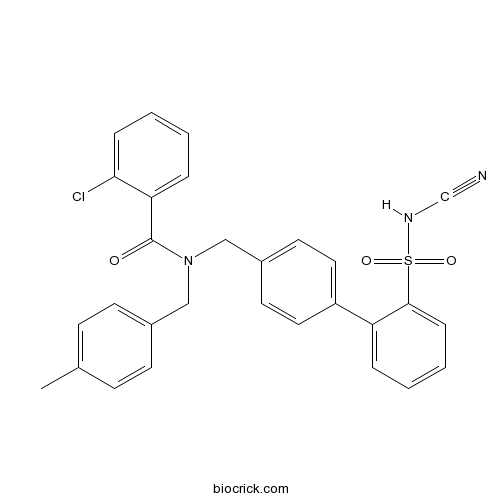
| Chemical Name | 2-chloro-N-[[4-[2-(cyanosulfamoyl)phenyl]phenyl]methyl]-N-[(4-methylphenyl)methyl]benzamide | ||
| SMILES | CC1=CC=C(C=C1)CN(CC2=CC=C(C=C2)C3=CC=CC=C3S(=O)(=O)NC#N)C(=O)C4=CC=CC=C4Cl | ||
| Standard InChIKey | ITDBPOSLOROLMT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H24ClN3O3S/c1-21-10-12-22(13-11-21)18-33(29(34)26-7-2-4-8-27(26)30)19-23-14-16-24(17-15-23)25-6-3-5-9-28(25)37(35,36)32-20-31/h2-17,32H,18-19H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | S0859, an N-cyanosulphonamide compound, reversibly inhibit NBC-mediated pH(i) recovery (K (i)=1.7 microM, full inhibition at approximately 30 microM).
IC50 value:
Target: NBC
Na(+)-coupled HCO(3)(-) transporters (NBCs) mediate the transport of bicarbonate ions across cell membranes and are thus ubiquitous regulators of intracellular pH. NBC dysregulation is associated with a range of diseases; for instance, NBCn1 is strongly up-regulated in a model of ErbB2-dependent breast cancer, a malignant and widespread cancer with no targeted treatment options, and single-nucleotide polymorphisms in NBCn1 genetically link to breast cancer development and hypertension. Treatment with NBC inhibitor S0859 significantly increased caspase-3 activity and elevated the number of apoptotic EC. S0859 is potentially important for probing the transporter's functional role in heart and other tissues. References: | |||||

S0859 Dilution Calculator

S0859 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8867 mL | 9.4333 mL | 18.8665 mL | 37.733 mL | 47.1663 mL |
| 5 mM | 0.3773 mL | 1.8867 mL | 3.7733 mL | 7.5466 mL | 9.4333 mL |
| 10 mM | 0.1887 mL | 0.9433 mL | 1.8867 mL | 3.7733 mL | 4.7166 mL |
| 50 mM | 0.0377 mL | 0.1887 mL | 0.3773 mL | 0.7547 mL | 0.9433 mL |
| 100 mM | 0.0189 mL | 0.0943 mL | 0.1887 mL | 0.3773 mL | 0.4717 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
S0859, an N-cyanosulphonamide compound, reversibly inhibited NBC-mediated pH(i) recovery (K (i)=1.7 microM, full inhibition at approximately 30 microM). Na(+)-coupled HCO(3)(-) transporters (NBCs) mediate the transport of bicarbonate ions across cell membranes and are thus ubiquitous regulators of intracellular pH. NBC dysregulation is associated with a range of diseases; for instance, NBCn1 is strongly up-regulated in a model of ErbB2-dependent breast cancer, a malignant and widespread cancer with no targeted treatment options, and single-nucleotide polymorphisms in NBCn1 genetically link to breast cancer development and hypertension. Treatment with NBC inhibitor S0859 significantly increased caspase-3 activity and elevated the number of apoptotic EC. S0859 is potentially important for probing the transporter's functional role in heart and other tissues.
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Regorafenib monohydrate
Catalog No.:BCC1884
CAS No.:1019206-88-2
- Dabigatran etexilate benzenesulfonate
Catalog No.:BCC8925
CAS No.:1019206-65-5
- sodium 4-pentynoate
Catalog No.:BCC1958
CAS No.:101917-30-0
- LX-4211
Catalog No.:BCC1714
CAS No.:1018899-04-1
- 7-Z-Trifostigmanoside I
Catalog No.:BCN7869
CAS No.:1018898-17-3
- Diclazuril
Catalog No.:BCC8937
CAS No.:101831-37-2
- Butenafine HCl
Catalog No.:BCC4768
CAS No.:101827-46-7
- TG 100801 Hydrochloride
Catalog No.:BCC1997
CAS No.:1018069-81-2
- Desacetylmatricarin
Catalog No.:BCN7258
CAS No.:10180-88-8
- 7-O-Demethyl-3-isomangostin hydrate
Catalog No.:BCN7882
CAS No.:
- Elliotinol
Catalog No.:BCN5833
CAS No.:10178-31-1
- Octacosyl (E)-ferulate
Catalog No.:BCN5834
CAS No.:101959-37-9
- Zardaverine
Catalog No.:BCC2069
CAS No.:101975-10-4
- GW791343 dihydrochloride
Catalog No.:BCC1613
CAS No.:1019779-04-4
- Acetoacetanilide
Catalog No.:BCC8803
CAS No.:102-01-2
- 3,4-Dihydroxyphenylacetic Acid
Catalog No.:BCC8281
CAS No.:102-32-9
- Phenyethyl 3-methylcaffeate
Catalog No.:BCN8457
CAS No.:71835-85-3
- Sulfaclozine
Catalog No.:BCC9155
CAS No.:102-65-8
- 20,24-Epoxy-24-methoxy-23(24-25)abeo-dammaran-3-one
Catalog No.:BCN1639
CAS No.:1020074-97-8
- SGI-1027
Catalog No.:BCC4588
CAS No.:1020149-73-8
- DCC-2036 (Rebastinib)
Catalog No.:BCC4390
CAS No.:1020172-07-9
- (R)-4-Benzyl-2-oxazolidinone
Catalog No.:BCC8395
CAS No.:102029-44-7
- PF-04457845
Catalog No.:BCC1851
CAS No.:1020315-31-4
Inhibition of monocarboxylate transporter by N-cyanosulphonamide S0859.[Pubmed:26027796]
Eur J Pharmacol. 2015 Sep 5;762:344-9.
The synthetic compound N-cyanosulphonamide S0859 has been described as a selective inhibitor of sodium-bicarbonate cotransporters (NBC, SLC4) in mammalian heart (Ch'en et al., 2008). First, for comparison, the electrogenic human NBCe1 (SLC4A4) was heterologously expressed in Xenopus laevis oocytes, where its transport activity was inhibited by S0859 with an IC50 of 9microM. The activity of monocarboxylate transporter (MCT) isoforms 1, 2, and 4 (SLC16A1, SLC16A7, SLC16A3), which transport lactate, pyruvate and ketone bodies, were also heterologously expressed in Xenopus oocytes, and their transport activity was similarly and reversibly inhibited by S0859 with an IC50 of 4-10microM. Partial inhibition of lactate transport by S0859 (50microM) was also obtained in cultured astrocytes of mice. Thus, S0859 appears to be an inhibitor of anion transport with a broader spectrum than previously thought, and may also interfere with cellular metabolite uptake/release.
S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart.[Pubmed:18204485]
Br J Pharmacol. 2008 Mar;153(5):972-82.
BACKGROUND AND PURPOSE: Intracellular pH (pH(i)) in heart is regulated by sarcolemmal H(+)-equivalent transporters such as Na(+)-H(+) exchange (NHE) and Na(+)-HCO(3) (-) cotransport (NBC). Inhibition of NBC influences pH(i) and can be cardioprotective in animal models of post-ischaemic reperfusion. Apart from a rabbit polyclonal NBC-antibody, a selective NBC inhibitor compound has not been studied. Compound S0859 (C(29)H(24)ClN(3)O(3)S) is a putative NBC inhibitor. Here, we provide the drug's chemical structure, test its potency and selectivity in ventricular cells and assess its suitability for experiments on cardiac contraction. EXPERIMENTAL APPROACH: pH(i) recovery from intracellular acidosis was monitored using pH-epifluorescence (SNARF-fluorophore) in guinea pig, rat and rabbit isolated ventricular myocytes. Electrically evoked cell shortening (contraction) was measured optically. With CO(2)/HCO(3) (-)-buffered superfusates containing 30 muM cariporide (to inhibit NHE), pH(i) recovery is mediated by NBC. KEY RESULTS: S0859, an N-cyanosulphonamide compound, reversibly inhibited NBC-mediated pH(i) recovery (K (i)=1.7 microM, full inhibition at approximately 30 microM). In HEPES-buffered superfusates, NHE-mediated pH(i) recovery was unaffected by 30 microM S0859. With CO(2)/HCO(3) (-) buffer, pH(i) recovery from intracellular alkalosis (mediated by Cl(-)/HCO(3) (-) and Cl(-)/OH(-) exchange) was also unaffected. Selective NBC-inhibition was not due to action on carbonic anhydrase (CA) enzymes, as 100 microM acetazolamide (a membrane-permeant CA-inhibitor) had no significant effect on NBC activity. pH(i) recovery from acidosis was associated with increased contractile-amplitude. The time course of recovery of pH(i) and contraction was slowed by S0859, confirming that NBC is a significant controller of contractility during acidosis. CONCLUSIONS AND IMPLICATIONS: Compound S0859 is a selective, high-affinity generic NBC inhibitor, potentially important for probing the transporter's functional role in heart and other tissues.
Gram-scale solution-phase synthesis of selective sodium bicarbonate co-transport inhibitor S0859: in vitro efficacy studies in breast cancer cells.[Pubmed:22927258]
ChemMedChem. 2012 Oct;7(10):1808-14.
Na(+)-coupled HCO(3)(-) transporters (NBCs) mediate the transport of bicarbonate ions across cell membranes and are thus ubiquitous regulators of intracellular pH. NBC dysregulation is associated with a range of diseases; for instance, NBCn1 is strongly up-regulated in a model of ErbB2-dependent breast cancer, a malignant and widespread cancer with no targeted treatment options, and single-nucleotide polymorphisms in NBCn1 genetically link to breast cancer development and hypertension. The N-cyanosulfonamide S0859 has been shown to selectively inhibit NBCs, and its availability on the gram scale is therefore of significant interest to the scientific community. Herein we describe a short and efficient synthesis of S0859 with an overall yield of 45 % from commercially available starting materials. The inhibitory effect of S0859 on recovery of intracellular pH after an acid load was verified in human and murine cancer cell lines in Ringer solutions. However, S0859 binds very strongly to components in plasma, and accordingly, measurements on isolated murine tissues showed no effect of S0859 at concentrations up to 50 muM.


