2-BenzoylpyridineCAS# 91-02-1 |
Quality Control & MSDS
Number of papers citing our products

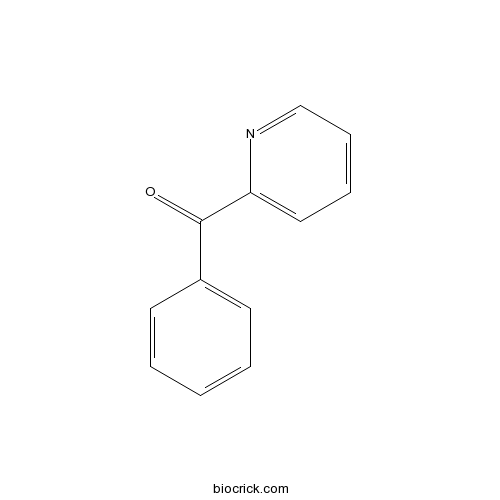
Chemical structure

3D structure
| Cas No. | 91-02-1 | SDF | Download SDF |
| PubChem ID | 7038 | Appearance | Powder |
| Formula | C12H9NO | M.Wt | 183 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | phenyl(pyridin-2-yl)methanone | ||
| SMILES | C1=CC=C(C=C1)C(=O)C2=CC=CC=N2 | ||
| Standard InChIKey | GCSHUYKULREZSJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H9NO/c14-12(10-6-2-1-3-7-10)11-8-4-5-9-13-11/h1-9H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2-Benzoylpyridine Dilution Calculator

2-Benzoylpyridine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4645 mL | 27.3224 mL | 54.6448 mL | 109.2896 mL | 136.612 mL |
| 5 mM | 1.0929 mL | 5.4645 mL | 10.929 mL | 21.8579 mL | 27.3224 mL |
| 10 mM | 0.5464 mL | 2.7322 mL | 5.4645 mL | 10.929 mL | 13.6612 mL |
| 50 mM | 0.1093 mL | 0.5464 mL | 1.0929 mL | 2.1858 mL | 2.7322 mL |
| 100 mM | 0.0546 mL | 0.2732 mL | 0.5464 mL | 1.0929 mL | 1.3661 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 8-Methoxybonducellin
Catalog No.:BCN4453
CAS No.:90996-27-3
- A 83-01
Catalog No.:BCC1319
CAS No.:909910-43-6
- W146
Catalog No.:BCC7723
CAS No.:909725-61-7
- α-CGRP (human)
Catalog No.:BCC5962
CAS No.:90954-53-3
- Cl-HIBO
Catalog No.:BCC7147
CAS No.:909400-43-7
- Erythrocentauric acid
Catalog No.:BCN7683
CAS No.:90921-13-4
- Broussonin E
Catalog No.:BCN4452
CAS No.:90902-21-9
- M871
Catalog No.:BCC5930
CAS No.:908844-75-7
- α-helical CRF 9-41
Catalog No.:BCC5727
CAS No.:90880-23-2
- Aprotinin
Catalog No.:BCC1220
CAS No.:9087-70-1
- Ptelatoside B
Catalog No.:BCN4451
CAS No.:90852-99-6
- ent-Labda-8(17),13E-diene-3beta,15,18-triol
Catalog No.:BCN7662
CAS No.:90851-50-6
- 2,6-Bis(hydroxymethyl)-p-cresol
Catalog No.:BCC8505
CAS No.:91-04-3
- Syringol
Catalog No.:BCN3534
CAS No.:91-10-1
- Coumarin
Catalog No.:BCN6309
CAS No.:91-64-5
- Benzoguanamine
Catalog No.:BCC8853
CAS No.:91-76-9
- N,N'-Bis(acetoacetyl)-o-toluidine
Catalog No.:BCC9062
CAS No.:91-96-3
- Fmoc-Arg-OH
Catalog No.:BCC3039
CAS No.:91000-69-0
- Impurity of Calcipotriol
Catalog No.:BCC5388
CAS No.:910133-69-6
- CGI-1746
Catalog No.:BCC1473
CAS No.:910232-84-7
- RGDS peptide
Catalog No.:BCC7694
CAS No.:91037-65-9
- BAY 60-6583
Catalog No.:BCC6197
CAS No.:910487-58-0
- Danshenol C
Catalog No.:BCN6681
CAS No.:910856-25-6
- 8-Epidiosbulbin E acetate
Catalog No.:BCN7812
CAS No.:91095-48-6
[Ag(L)NO3] Complexes with 2-Benzoylpyridine-Derived Hydrazones: Cytotoxic Activity and Interaction with Biomolecules.[Pubmed:30221236]
ACS Omega. 2018 Jun 30;3(6):7027-7035.
Complexes [Ag(H2BzPh)NO3] (1), [Ag(H2BzpCH3Ph)NO3] (2), [Ag(H2BzpClPh)NO3] (3), and [Ag(H2BzpNO2Ph)NO3] (4) were synthesized with 2-Benzoylpyridine benzoylhydrazone (H2BzPh) and its para-methyl-benzoylhydrazone (H2BzpCH3Ph), para-chloro-benzoylhydrazone (H2BzpClPh), and para-nitro-benzoylhydrazone (H2BzpNO2Ph) derivatives. Experimental data indicate that the nitrate ligand binds more strongly to the silver center through one of the oxygen atoms, whereas the second oxygen atom from nitrate and the hydrazone oxygen makes much weaker interactions with the metal. Dissociation of nitrate most probably occurs in solution and in biological media. Interestingly, theoretical calculations suggested that when dissociation of the nitrate takes place, all bond orders involving the metal and the atoms from the hydrazone ligand increase significantly, showing that the bonding of nitrate results in the weakening of all other interactions in the metal coordination sphere. Upon complexation of the hydrazones to silver(I), cytotoxicity against B16F10 metastatic murine melanoma cells increased in all cases. Complexes (1-3) proved to be more cytotoxic than cisplatin. All compounds were more cytotoxic to B16F10 cells than to nontumorigenic murine Melan-A melanocyte cells. Interestingly, the selectivity index (SI = IC50 non-malignant cells/IC50 tumor cells) of complex (1), SI = 23, was much higher than that of the parent hydrazone ligand, SI = 9.5. Studies on the interactions of complexes (1-3) with DNA suggested that although (1-3) interact with calf thymus DNA by an intercalative mode, direct covalent binding of silver(I) to DNA probably does not occur. Complexes (1-3) interact in vitro with human serum albumin indicating that these compounds could be transported by albumin.
2-Benzoylpyridine Ligand Complexation with Gold Critical for Propargyl Ester-Based Protein Labeling.[Pubmed:29791049]
Chemistry. 2018 Jul 25;24(42):10595-10600.
In previously reported work, Au(III) complexes coordinated with 2-Benzoylpyridine ligand, BPy-Au, were prebound to a protein and used to discover a novel protein-directed labeling approach with propargyl ester functional groups. In this work, further examination discovered that gold catalysts devoid of the 2-Benzoylpyridine ligand (e.g., NaAuCl4) had significantly reduced levels of protein labeling. Mechanistic investigations then revealed that BPy-Au and propargyl esters undergo a rare example of C(sp(2) )-C(sp) aryl-alkynyl cross-coupling, likely through spontaneous reductive elimination. Overall, these observations appear to suggest that BPy-Au-mediated, propargyl ester-based protein labeling acts via an activated ester intermediate, which contributes to our understanding of this process and will aid the expansion/optimization of gold-catalyst usage in future bioconjugation applications, especially in vivo.
Structure-Activity Relationships of Di-2-pyridylketone, 2-Benzoylpyridine, and 2-Acetylpyridine Thiosemicarbazones for Overcoming Pgp-Mediated Drug Resistance.[Pubmed:27524608]
J Med Chem. 2016 Sep 22;59(18):8601-20.
Multidrug resistance (MDR) mediated by P-glycoprotein (Pgp) represents a significant impediment to successful cancer treatment. The compound, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT), has been shown to induce greater cytotoxicity against resistant cells than their nonresistant counterparts. Herein, the structure-activity relationships of selected thiosemicarbazones are explored and the novel mechanism underlying their ability to overcome resistance is further elucidated. Only thiosemicarbazones with electron-withdrawing substituents at the imine carbon mediated Pgp-dependent potentiated cytotoxicity, which was reversed by Pgp inhibition. Treatment of resistant cells with these thiosemicarbazones resulted in Pgp-dependent lysosomal membrane permeabilization (LMP) that relied on copper (Cu) chelation, reactive oxygen species generation, and increased relative lipophilicity. Hence, this study is the first to demonstrate the structural requirements of these thiosemicarbazones necessary to overcome MDR. We also demonstrate the mechanism that enables the targeting of resistant tumors, whereby thiosemicarbazones "hijack" lysosomal Pgp and form redox-active Cu complexes that mediate LMP and potentiate cytotoxicity.


