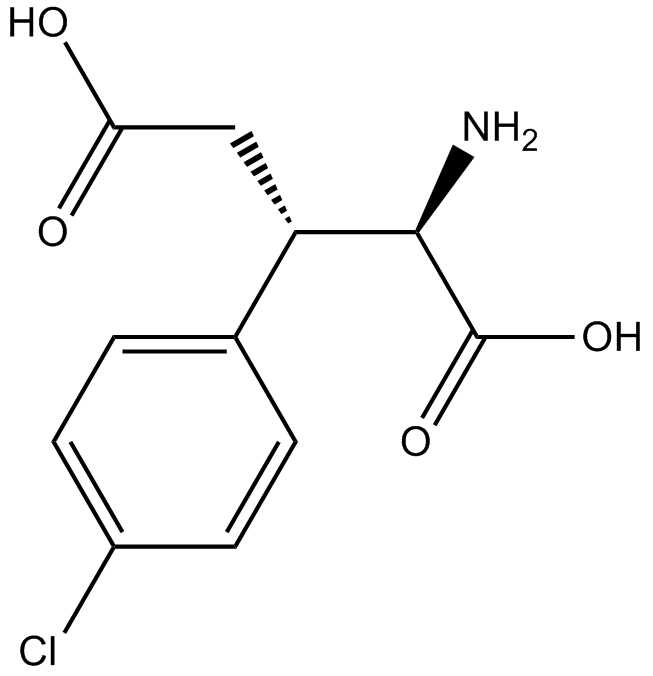
(2R,3S)-ChlorphegCAS# 140924-23-8 |
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 140924-23-8 | SDF | Download SDF |
| PubChem ID | 140924-23-8 | Appearance | Powder |
| Formula | C11H12ClNO4 | M.Wt | 257.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1.1eq. NaOH | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A weakly active NMDA receptor antagonist. |

(2R,3S)-Chlorpheg Dilution Calculator

(2R,3S)-Chlorpheg Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8809 mL | 19.4047 mL | 38.8093 mL | 77.6187 mL | 97.0233 mL |
| 5 mM | 0.7762 mL | 3.8809 mL | 7.7619 mL | 15.5237 mL | 19.4047 mL |
| 10 mM | 0.3881 mL | 1.9405 mL | 3.8809 mL | 7.7619 mL | 9.7023 mL |
| 50 mM | 0.0776 mL | 0.3881 mL | 0.7762 mL | 1.5524 mL | 1.9405 mL |
| 100 mM | 0.0388 mL | 0.194 mL | 0.3881 mL | 0.7762 mL | 0.9702 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Hydroxy-5,6-dehydrosugiol
Catalog No.:BCN3127
CAS No.:140923-35-9
- 4-Chloro-D-phenylalanine
Catalog No.:BCC2637
CAS No.:14091-08-8
- Aminopotentidine
Catalog No.:BCC6761
CAS No.:140873-26-3
- ICI 215,001 hydrochloride
Catalog No.:BCC5688
CAS No.:140850-02-8
- 1-Hydroxymethyl-beta-carboline glucoside
Catalog No.:BCN7026
CAS No.:1408311-12-5
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- PF 06465469
Catalog No.:BCC6268
CAS No.:1407966-77-1
- Scandine Nb-oxide
Catalog No.:BCN7504
CAS No.:140701-69-5
- 4-Aza-5androstan-1-ene- 3-one-17carboxylic acid
Catalog No.:BCC8693
CAS No.:140700-63-6
- 1-Deoxydihydroceramide-1-sulfonic acid
Catalog No.:BCC4964
CAS No.:1407-03-0
- Levatin
Catalog No.:BCN2531
CAS No.:140670-84-4
- Kadsurenin D
Catalog No.:BCN6603
CAS No.:140669-89-2
- Benzo-18-crown-6 ether
Catalog No.:BCC8850
CAS No.:14098-24-9
- Benzo-15-crown 5-ether
Catalog No.:BCC8849
CAS No.:14098-44-3
- Nerylacetate
Catalog No.:BCN3802
CAS No.:141-12-8
- Ricinoleic acid
Catalog No.:BCC8248
CAS No.:141-22-0
- 2-Aminoethanol
Catalog No.:BCN1756
CAS No.:141-43-5
- Malonic acid
Catalog No.:BCN8534
CAS No.:141-82-2
- 2-Thiouracil
Catalog No.:BCC4752
CAS No.:141-90-2
- Frangulin B
Catalog No.:BCC8175
CAS No.:14101-04-3
- Gama-Tocotrienol
Catalog No.:BCN3720
CAS No.:14101-61-2
- PACOCF3
Catalog No.:BCC7074
CAS No.:141022-99-3
- 1-Hydroxy-2,3,4,7-tetramethoxyxanthone
Catalog No.:BCN6506
CAS No.:14103-09-4
- Xanomeline oxalate
Catalog No.:BCC4146
CAS No.:141064-23-5
Differential actions of 3-(4-chlorophenyl) glutamic acid stereoisomers and L-trans-pyrrolidine-2,4-dicarboxylic acid upon L-homocysteic acid- and L-glutamic acid-induced responses from rat spinal motoneurones.[Pubmed:8788956]
Neuropharmacology. 1995 Dec;34(12):1589-95.
The four recently synthesized stereoisomers of 3-(4-chlorophenyl) glutamic acid (chlorpheg) were individually examined for their abilities to potentiate depolarizations of neonatal rat motoneurones evoked by L-homocysteic acid (L-HCA, 10 microM). This property had previously been observed using the racemate and is believed to be mediated by uptake inhibition. Both the (2S,3S)- and (2S,3R)- isomers were selective potentiators of L-HCA- (vs L-Glu) induced depolarizations although the (2S,3S)- isomer was more effective. The (2R,3S)- isomer had a slight but significant depressant action which could be attributed to N-methyl-D-aspartate (NMDA) receptor antagonism. Comparison of the potentiating properties of (2S,3S)- and (2S,3R)-chlorpheg with those of L-trans-pyrrolidine-2,4-dicarboxylic acid (tPDC, a L-Glu uptake inhibitor) upon L-HCA- and L-Glu-evoked responses revealed that both chlorpheg isomers (500 microM each) selectively potentiated responses evoked by L-HCA (10 microM) but had no significant effect upon those evoked by L-Glu (50 microM). On the other hand, use of tPDC at the same concentration significantly enhanced the depolarizations evoked by both amino acids, although its action on L-Glu-evoked responses was greater. It is concluded that (i) the (2S,3S)- isomer and to a lesser extent, the (2S,3R)- isomer of chlorpheg are responsible for the potentiating actions seen with the chlorpheg racemate used in previous studies and (ii) (2R,3S)-Chlorpheg is a weak NMDA antagonist. The apparently selective action of (2S,3S)- and (2S,3R)-chlorpheg upon L-HCA-relative to L-Glu-induced depolarizations supports the existence of multiple excitatory amino acid uptake sites, some of which may yet be unidentified.
Diastereoselective synthesis of all four isomers of 3-(4-chlorophenyl) glutamic acid: identification of the isomers responsible for the potentiation of L-homocysteic acid-evoked depolarizations in neonatal rat motoneurons.[Pubmed:8941386]
J Med Chem. 1996 Nov 22;39(24):4738-43.
All four isomers of 3-(4-chlorophenyl)glutamic acid (5-8) were prepared by diastereoselective synthesis. Addition of (6S)-(+)-bis-lactim ether 15 to cis-4-chlorocinnamate 12 gave a mixture comprising mainly the (2R,3S)- and (2R,3R)-isomers 5 and 6, respectively (in a ratio of 56:40), while addition of (6R)-(-)-bis-lactim ether 16 to 4-chlorocinnamate 12 gave a mixture comprising mainly the (2S,3R)- and (2S,3S)-isomers 8 and 7, respectively (in a ratio of 56:42). The four stereoisomers (5-8) were therefore conveniently prepared by addition of either 3-lithio-(6S)- or -(6R)-bis-lactim ether (15 or 16, respectively) to 4-chlorocinnamate 12 and separation of the resultant mixtures of diastereoisomers (23-26) by flash silica gel chromatography. The absolute configurations of 6 and 7 were confirmed by X-ray crystallography. Both the (2S,3S)- and (2S,3R)-isomers (7 and 8, respectively) at a concentration of 100 microM significantly potentiated depolarizations induced by 10 microM L-homocysteic acid (L-HCA) (% control +/- sem: 130.4 +/- 3.6, n = 20 and 114.5 +/- 2.4, n = 11, respectively) while the (2R,3S)-isomer 5 significantly reduced L-HCA response amplitude (94.2 +/- 1.4, n = 9) and the (2R,3R)-isomer 6 was inactive. Experiments designed to compare the agonist-potentiating actions of 7 and 8 in the neonatal rat spinal cord with L-trans-pyrrolidine-2,4-dicarboxylic acid, the well-known L-Glu uptake inhibitor, provided additional evidence for the selective enhancement of depolarizations due to L-HCA and not those due to L-Glu. This selective action supports the existence of multiple excitatory amino acid uptake sites.


