Total synthesis of the sesquiterpene agarozizanol B
Using available chemicals, a team of German researchers has achieved the total synthesis of agarozizanol B, an interesting natural substance found in agarwood. As described in the journal Angewandte Chemie, the key sequence in this relatively short synthetic pathway is a cascade of photochemical reactions that involves a series of complex rearrangements of polycyclic intermediates.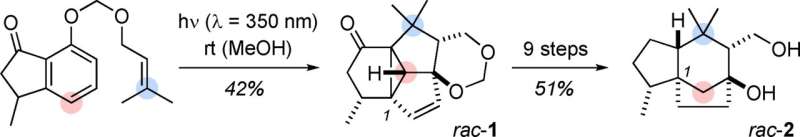
Terpenes are natural substances that originate mainly from plants and show a great structural diversity. Formally, they consist of isoprene units (2-methylbuta-1,3-diene). Sesquiterpenes, terpenes composed of three isoprene units, are the largest and most important subset. Their basic structure consists of 15 carbon atoms that can be arranged in many different ways, usually in ring systems of several carbon atoms. Some polycyclic sesquiterpenes are used as fragrant and aromatic compounds, as well as pharmaceutical agents.
A family of sesquiterpenes with antidiabetic activity and a high degree of structural complexity has recently been isolated from agarwood (also known as eaglewood) and several other plants. They all share a common central structure known as the prezizan skeleton. Under the leadership of Thorsten Bach of the Technical University of Munich, Germany, a team has now completed the total synthesis of one member of this family of natural products, agarozizanol B. A total synthesis is the complete formation of a natural product from obtainable basic raw materials.
The backbone of prezizane is a system of three rings of carbon atoms: a six-membered ring that is attached to a five-membered ring along one edge and is also bridged by two other carbon atoms to form a second five-membered ring. The difficulty in synthesizing such a structure lies in the fact that the backbone can exist in two symmetrical forms (called (+) and (-) forms). Agarozizanol B also has two side groups that must also have the correct spatial orientation.
The starting point for this eleven-step reaction sequence - which is very short for a total synthesis - is an indanone derivative. This compound already contains a system with a five-membered ring and a six-membered, but aromatic ring. The missing carbon atoms are attached to the indanone in the form of an olefinic side chain. At the heart of this synthesis is a photochemical cascade that involves three light-triggered reactions and a series of complex rearrangements of polycyclic intermediates. The first step produces a taut tetracyclic framework that contains all the carbon atoms of agarozizanol B in the correct relative positions, with the loss of the unwanted aromaticity of the six-carbon ring. Later, a conversion leads to the tricyclic skeleton of prezizane by opening a three-carbon ring in an intermediate (splitting a cyclopropane bond).
"In this way, we obtained a mixture of the (+) and (-) forms as well as pure (+)-agarozizanol B, which is identical to the natural product," explains Thorsten Bach. "Our newly developed photochemical cascade reaction has shown great promise for future syntheses of other natural products."


