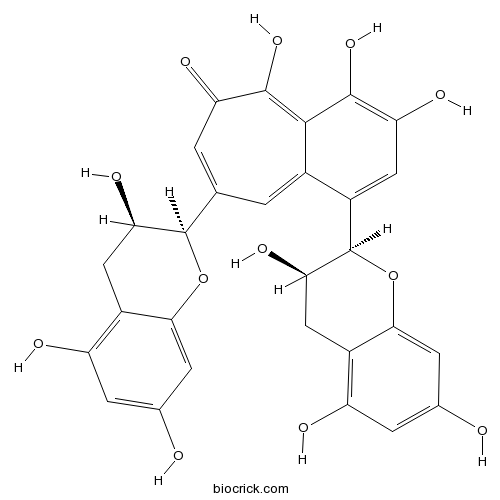
A biflavonoid that is 3,4,5-trihydroxybenzocyclohepten-6-one which is substituted at positions 1 and 8 by (2R,3R)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-2-yl groups. It is the main red pigment in black tea.
InChI=1S/C29H24O12/c30-11-3-17(32)15-8-21(36)28(40-23(15)5-11)10-1-13-14(7-20(35)27(39)25(13)26(38)19(34)2-10)29-22(37)9-16-18(33)4-12(31)6-24(16)41-29/h1-7,21-22,28-33,35-37,39H,8-9H2,(H,34,38)/t21-,22-,28-,29-/m1/s1
Theaflavins(TF) has antioxidant activity, the TF present in black tea possess at least the same antioxidant potency as catechins present in green tea, and that the conversion of catechins to TF during fermentation in making black tea does not alter significantly their free radical-scavenging activity.[1,2]
Theaflavins significantly reduce lipid accumulation, suppressed fatty acid synthesis, and stimulate fatty acid oxidation, also inhibits acetyl-coenzyme A carboxylase activities by stimulating AMP-activated protein kinase (AMPK) through the LKB1 and reactive oxygen species pathways; it is bioavailable both in vitro and in vivo and may be active in the prevention of fatty liver and obesity.[3]
Theaflavins induce G2/M arrest by modulating expression of p21 waf1/cip1 , cdc25C and cyclin B in human prostate carcinoma PC-3 cells, it acts as an effective anti-proliferative agent by modulating cell growth regulators in prostate cancer cells.[4]
Theaflavins from Black Tea, have inhibition of ultraviolet B&ndash and induces AP‐1 activation.[5]
Theaflavins inhibit 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in A/J mice.[6]
Theaflavins and thearubigins have antimutagenic effects in Ames Salmonella assays.[7]
English website: Theaflavin
Japanese website: Theaflavin
Chinese website: Theaflavin
[1] Leung L K, Su Y, Chen R, et al. J Nut, 2001, 131(9):2248-51.
[2] Lin C L, Huang H C, Lin J K. J Lipid Res, 2007, 48(11):2334-43.
[3] Masaaki N, Ma W, Huang C, et al. Mol Carcinogen, 2000, 28:148-55.
[4] Prasad S, Kaur J, Roy P, et al. Life Sci, 2007, 81(17-18):1323-31.
[5] Yang G Y, Liu Z, Seril D N, et al. Carcinogenesis, 1997, 18(12):2361-5.
[7] Gupta S, Chaudhuri T, Seth P, et al. Phytother Res, 2002, 16(7):655-61.
[8] Kim S Y, Kozukue N, Han J S, et al. 한국식품과학회지제38권제1호, 2006, 38(1):5-9.