Daphne giraldii
Daphne giraldii
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Daphne giraldii
- Cat.No. Product Name CAS Number COA
-
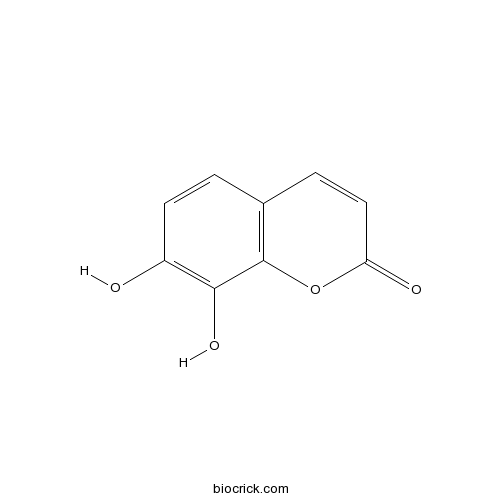
BCN1051
Daphnetin486-35-1
Instructions
Identification of three Daphne species by DNA barcoding and HPLC fingerprint analysis.[Pubmed: 30071090]
In order to well identify the 93 wild Cortex Daphnes samples from different species and habitats in western China and develop a standard operating procedure (SOP) for the authentication and quality of them in the future, a comprehensive and efficient identification system based on DNA barcoding and HPLC fingerprint technologies has been developed. The result showed that only 17 samples (18%) were Daphne giraldii Nitsche (DG), which is recorded in Chinese Pharmacopeia, while the others (82%) might have safety hazards. Additionally, the result of HPLC fingerprint analysis indicated that samples in the same species origins and wild distributions could be clustered together, which was consistent with DNA barcoding analysis. The study can provide a significant system for the authentication and quality of commercial Cortex Daphnes herbs. Undoubtedly, this study undoubtedly confirmed that the chemical compositions of Cortex Daphnes herbs were affected by both species origins and ecological environments, which is required more in-depth research.
Flavan enantiomers from Daphne giraldii selectively induce apoptotic cell death in p53-null hepatocarcinoma cells in vitro.[Pubmed: 29698619]
None
1,3-Diphenylpropanes from Daphne giraldii induced apoptosis in hepatocellular carcinoma cells through nuclear factor kappa-B inhibition.[Pubmed: 29501029]
None
Simultaneous determination and pharmacokinetic study of giraldoid a, giraldoid B in rat plasma after oral administration of Daphne giraldii Nitsche extracts by LC-MS/MS.[Pubmed: 29110318]
None
[Simultaneous determination of daphnetin, daphnoretin, daphneticin in rat plasma by LC-MS/MS and its application in pharmacokinetic study].[Pubmed: 29090558]
To establish HPLC-MS/MS method for simultaneous determination of daphnetin, daphnoretin, and daphneticin in rat plasma after oral and intravenous administration of Daphne giraldii extract, and then use them in the calculation of pharmacokinetic parameters. Six sprague-dawley rats received intragastric administration of D. giraldii extract (daphnetin, daphnoretin and daphneticin were 88.40, 3.24 and 4.28 mg•kg⁻¹, respectively). Their drug plasma concentration was determined by LC-MS/MS with schisandrin as an internal standard to draw plasma concentration-time curve. The pharmacokinetic parameters were calculated by Kinetica 4.4. The results showed that the linear range was 5-1 000 μg•L⁻¹ for daphnetin, daphnoretin and daphneticin, and the method ological test showed conformance to the requirements.The intraday and inter-day variable coefficients (RSD) were both less than 15.0%, indicating that both of legitimate precise and accuracy were consistent with the analysis requirements of biological samples. For daphnetin, the pharmacokinetic parameters Tmax, Cmax, AUC0-t, T1/2 and MRT were 4 h, 858.96 μg•L⁻¹, 10 566.4 μg•L⁻¹•h, 5.19 h and 9.43 h, respectively. For daphnoretin, the pharmacokinetic parameters Tmax, Cmax, AUC0-t, T1/2 and MRT were 2.92 h, 178.00 μg•L⁻¹, 905.89 μg•L⁻¹•h, 3.50 h and 6.95 h, respectively. For daphneticin, the pharmacokinetic parameters Tmax, Cmax, AUC0-t, T1/2 and MRT were 2 h, 36.67 μg•L⁻¹, 355.11 μg•L⁻¹•h, 4.95 h and 8.27 h, respectively. The LC-MS/MS analysis method established in this study was proved to be so accurate and sensitive that it can be applied to the pharmacokinetic study of daphnetin, daphnoretin and daphneticin.
A new Prenylated Flavonoid induces G0/G1 arrest and apoptosis through p38/JNK MAPK pathways in Human Hepatocellular Carcinoma cells.[Pubmed: 28720813]
Prenylated flavonoids have been demonstrated to possess diverse bioactivities including antitumor effects. One new, daphnegiravone D (1), and four known (2-5) prenylated flavonoids were isolated from Daphne giraldii. Their cytotoxic activities revealed that daphnegiravone D markedly inhibited the proliferation of cancer cells, but had no apparent cytotoxicity on human normal cells. Mechanistically, daphnegiravone D induced G0/G1 arrest and apoptosis, reduced the expression of cyclin E1, CDK2 and CDK4, and promoted the cleavage of caspase 3 and PARP in Hep3B and HepG2 cells. Meanwhile, daphnegiravone D increased the level of phosphorylated p38 and attenuated phosphorylated JNK. Further studies indicated that SB203580 partially reversed daphnegiravone D-induced G0/G1 arrest and apoptosis. The addition of SP600125 to both cell lines increased the cleavage of caspase 3 and PARP, but did not affect the G0/G1 arrest. Besides, in vivo studies demonstrated that daphnegiravone D obviously inhibited tumor growth in a nude mouse xenograft model through suppressing the proliferation of tumor cells, without significant effect on body weight or pathology characteristics. Taken together, the new compound selectively inhibited the proliferation of hepatoma cells via p38 and JNK MAPK pathways, suggesting its potential as a novel natural anti-hepatocellular carcinoma agent.
Autophagy antagonizes apoptosis induced by flavan enantiomers from Daphne giraldii in hepatic carcinoma cells in vitro.[Pubmed: 28371676]
None
Chiral Separation of Cytotoxic Flavan Derivatives from Daphne giraldii.[Pubmed: 27627130]
Twelve new flavan derivatives including four pairs of enantiomers, daphnegiralins A1-A4 (1) and daphnegiralins B1-B4 (2), and two pairs of epimers, daphnegiralins C1/C2 (3) and daphnegiralins D1/D2 (4), were isolated from the stem bark and roots of Daphne giraldii. Their structures were elucidated using spectroscopic analyses, computational approaches, and chemical methods. Separation of the enantiomeric mixtures (1a, 1b, 2a, and 2b) was achieved using chiral HPLC. The compounds were evaluated against a small panel of human cancer cell lines, and 1b-2, 2a, and 2b were cytotoxic against Hep3B human hepatoma cells.
Cytotoxic prenylated flavones from the stem and root bark of Daphne giraldii.[Pubmed: 27400887]
Three new prenylated flavones (1-3), along with three known analogues (4-6), were isolated from the stem and root bark of Daphne giraldii. Their structures were determined by comprehensive NMR and HRESIMS spectroscopic data analyses. The absolute configurations of compounds 2 and 3 were assigned by optical rotation comparison, CD and [Rh2(OCOCF3)4]-induced CD spectral methods. The in vitro cytotoxicity experiments carried out involving five cancer cell lines (U251, A549, HepG2, MCF-7 and Bcap37) showed that 2 markedly inhibited the proliferation of all tested cells with IC50 values ranging from 4.26 to 20.82μM. The preliminary structure-activity relationships of these flavones are discussed. In addition, compound 2 was found to effectively induce apoptosis in HepG2 cells according to a flow cytometry analysis.
Chemical Constituents from Daphne giraldii Nitsche and Their Contents Simultaneous Determination by HPLC.[Pubmed: 27190543]
Daphne giraldii Nitsche (Thymelaeaceae) is widely distributed in the Chinese provinces of Shaanxi, Gansu, and Qinghai, which has been used in Chinese folk medicine to treat ache and rheumatism. Pharmacologic tests have revealed that the plant has anti-inflammatory, analgesic, and anticancer activities. However, there is still not enough systemic investigation on the chemical constituents and the method for the contents simultaneous determination in D. giraldii. Therefore, the isolation and characterization of the compounds from the stem barks of this plant were reported. Moreover, a facile, accurate, and reliable method has been developed and validated for their simultaneous determination using HPLC-DAD.


