Clematoclethra scandens subsp. actinidioides
Clematoclethra scandens subsp. actinidioides
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Clematoclethra scandens subsp. actinidioides
- Cat.No. Product Name CAS Number COA
-
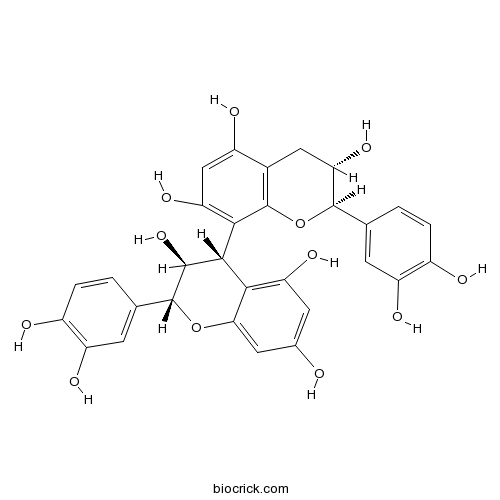
BCN6316
Procyanidin B323567-23-9
Instructions
-
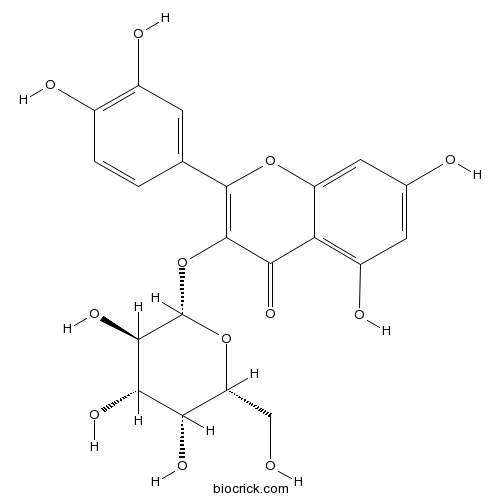
BCN5570
Hyperoside482-36-0
Instructions
A new ursane-type triterpenoid saponin from the aerial parts of Clematoclethra scandens subsp. actinidioides.[Pubmed: 25660290]
A new ursane-type triterpenoid saponin, 2α,3α,24-trihydroxyurs-12,20(30)-dien-28-oic acid β-D-glucopyranosyl ester (1), together with six known triterpenoid saponins, was isolated and characterized from the aerial parts of Clematoclethra scandens subsp. actinidioides.
[Triterpenes from aerial parts of Clematoclethra scandens subsp. actinidioides].[Pubmed: 23668009]
To study the chemical constituents of Clematoclethra scandens subsp. actinidioides, chromatographic methods such as silica gel and MCI column chromatographic technology, and preparative HPLC were used and sixteen compounds were isolated from the aerial parts of this plant. By using spectroscopic techniques including 1H, 13C-NMR, HMBC and ESI-MS, these compounds were identified as betulinic acid (1), ursolic acid (2), oleanic acid (3), corosolic acid (4), 3beta-(trans-p-coumaroyloxy)-2alpha, 23-dihydroxyurs-12-en-28-oic acid (5), 3beta-(trans-p-coumaroyloxy)-2alpha, 23-dihydroxyurs-12, 20 (30)-dien-28-oic acid (6), 2alpha, 3alpha, 23-trihydroxyurs-12, 20 (30)-dien-28-oic acid (7), 2alpha, 3alpha, 23-trihydroxyurs-12-en-28-oic acid (8), asiatic acid (9), 2alpha, 3alpha, 24-tri-hydroxyurs-12-en-28-oic acid (10), 2alpha, 3beta, 23-trihydroxyurs-12, 20 (30)-dien-28-oic acid (11), 2alpha, 3beta, 19alpha, 24-tetrahydroxyurs-12-en-28-oic acid (12), 2alpha, 3alpha, 19alpha, 24-tetrahydroxyurs-12-en-28-oic acid (13), 2alpha, 3beta, 23, 24-tetrahydroxyurs-12-en-28-oic acid (14), 2alpha, 3alpha, 19alpha, 23, 24-pentahydroxyurs-12-en-28-oic acid (15) and daucosterol (16). Among them, compounds 3-6, 11-12, 14 and 15 were isolated from this endemic plant for the first time.


