Cinnamomum subavenium
Cinnamomum subavenium
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Cinnamomum subavenium
- Cat.No. Product Name CAS Number COA
-
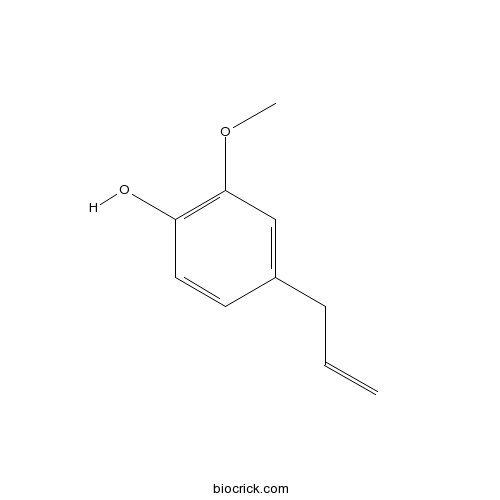
BCN5964
Eugenol97-53-0
Instructions
Synergistic Effects of Linderanolide B Combined with Arbutin, PTU or Kojic Acid on Tyrosinase Inhibition.[Pubmed: 26343134]
Melanin uncontrollable accumulation is a serious social problem to not only women, but also men, and causes pigment over-expression disorders such as freckles, melasma or pigmented acne scars. The synergism is used widely in medication, and the effectiveness makes the drug applications more valuable. Within this experiment, three well-known compounds were chosen: kojic acid, 1-phenyl-2-thiourea (PTU) and arbutin, and they were combined individually with our substance linderanolide B, which is purified from Cinnamomum subavenium. Hence, deciphering the synergistic action of possible whitening agents was the goal of this study. The tyrosinase activity, melanin content, and the combination index (CI) values were observed in B16F10 cells, in addition, the consequences were detected by isobologram analysis. We discovered that certain melanin inhibitors showed synergistic properties when they were combined together to suppress tyrosinase activities. As a result, linderanolide B has a potential synergy on tyrosinase inhibition, and it can be used widely in cosmetic and medication industries.
Neolignans with a Rare 2-Oxaspiro[4.5]deca-6,9-dien-8-one Motif from the Stem Bark of Cinnamomum subavenium.[Pubmed: 26087384]
Two pairs of racemic spirodienone neolignans with a rare 2-oxaspiro[4.5]deca-6,9-dien-8-one motif, named (±)-subaveniumins A (1) and B (2), were isolated from the bark of Cinnamomum subavenium. The chiral separation of the (+)-1, (-)-1, (+)-2, and (-)-2 enantiomers was accomplished via high-performance liquid chromatography on a chiral column. Their structures were elucidated using single-crystal X-ray diffraction and spectroscopic analyses (UV, IR, HRESIMS, and 1D and 2D NMR). The absolute configurations of the enantiomers were determined by comparing the experimental and calculated electronic circular dichroic spectra. The (+)-1, (-)-1, (+)-2, and (-)-2 enantiomers exhibited moderate inhibitory effects against NO production in RAW264.7 mouse macrophages induced by lipopolysaccharide, with IC50 values of 17.9, 5.6, 15.1, and 4.3 μM, respectively.
Subamolide B Isolated from Medicinal Plant Cinnamomum subavenium Induces Cytotoxicity in Human Cutaneous Squamous Cell Carcinoma Cells through Mitochondrial and CHOP-Dependent Cell Death Pathways.[Pubmed: 23573140]
Subamolide B is a butanolide isolated from Cinnamomum subavenium, a medicinal plant traditionally used to treat various ailments including carcinomatous swelling. We herein reported for the first time that subamolide B potently induced cytotoxicity against diverse human skin cancer cell lines while sparing nonmalignant cells. Mechanistic studies on human cutaneous squamous cell carcinoma (SCC) cell line SCC12 highlighted the involvement of apoptosis in subamolide B-induced cytotoxicity, as evidenced by the activation of caspases-8, -9, -4, and -3, the increase in annexin V-positive population, and the partial restoration of cell viability by cotreatment with the pan-caspase inhibitor z-VAD-fmk. Additionally, subamolide B evoked cell death pathways mediated by FasL/Fas, mitochondria, and endoplasmic reticulum (ER) stress, as supported by subamolide B-induced FasL upregulation, BCL-2 suppression/cytosolic release of cytochrome c, and UPR activation/CHOP upregulation, respectively. Noteworthy, ectopic expression of c-FLIPL or dominant-negative mutant of FADD failed to impair subamolide B-induced cytotoxicity, whereas BCL-2 overexpression or CHOP depletion greatly rescued subamolide B-stimulated cells. Collectively, these results underscored the central role of mitochondrial and CHOP-mediated cell death pathways in subamolide B-induced cytotoxicity. Our findings further implicate the potential of subamolide B for cutaneous SCC therapy or as a lead compound for developing novel chemotherapeutic agents.
Subamolide a induces mitotic catastrophe accompanied by apoptosis in human lung cancer cells.[Pubmed: 23533526]
This study investigated the anticancer effects of subamolide A (Sub-A), isolated from Cinnamomum subavenium, on human nonsmall cell lung cancer cell lines A549 and NCI-H460. Treatment of cancer cells with Sub-A resulted in decreased cell viability of both lung cancer cell lines. Sub-A induced lung cancer cell death by triggering mitotic catastrophe with apoptosis. It triggered oxidant stress, indicated by increased cellular reactive oxygen species (ROS) production and decreased glutathione level. The elevated ROS triggered the activation of ataxia-telangiectasia mutation (ATM), which further enhanced the ATF3 upregulation and subsequently enhanced p53 function by phosphorylation at Serine 15 and Serine 392. The antioxidant, EUK8, significantly decreased mitotic catastrophe by inhibiting ATM activation, ATF3 expression, and p53 phosphorylation. The reduction of ATM and ATF3 expression by shRNA decreased Sub-A-mediated p53 phosphorylation and mitotic catastrophe. Sub-A also caused a dramatic 70% reduction in tumor size in an animal model. Taken together, cell death of lung cancer cells in response to Sub-A is dependent on ROS generation, which triggers mitotic catastrophe followed by apoptosis. Therefore, Sub-A may be a novel anticancer agent for the treatment of nonsmall cell lung cancer.
Dibenzocycloheptanoids from the leaves of Cinnamomum subavenium.[Pubmed: 23025417]
Five dibenzocycloheptatrienes (1-3, 5, and 6) and one dibenzocycloheptadiene (8) together with 16 known compounds were isolated from the leaves of Cinnamomum subavenium. Application of HPLC-SPE-NMR to a selected fraction afforded two additional dibenzocycloheptatrienes (4, 7). The glycosides 2-7 comprise two diastereomers because of the chiral glycosyl moiety and the axial chirality of the biphenyl system. Their structures were elucidated via ECD and 2D NMR analyses and chemical degradation. Subavenosides D (5) and E (6) showed moderate inhibitory activity against α-glucosidase type IV from Bacillus stearothermophilus with IC₅₀ values of 50.7 and 19.0 μM, respectively.
Review on pharmacological activities of Cinnamomum subavenium.[Pubmed: 22691063]
This review describes the morphological, phytochemical and pharmacological properties of Cinnamomum subavenium (Lauraceae). The plant grows wild in southern Mainland China, Burma, Cambodia, Taiwan, Malaysia and Indonesia. This plant is recorded as having long been used to treat carcinomatous swelling, stomach ache, chest pain, abdominal pain, hernia, diarrhoea, rheumatism, nausea and vomiting. This article enumerates an overview of phytochemical and pharmacological aspects that is useful to researchers for further exploration for the necessary development of this potential herb.
Subamolide A, a component isolated from Cinnamomum subavenium, induces apoptosis mediated by mitochondria-dependent, p53 and ERK1/2 pathways in human urothelial carcinoma cell line NTUB1.[Pubmed: 21708241]
Cinnamomum subavenium has long been used as a traditional Chinese medicine to treat carcinomatous swelling, abdominal pain and other diseases.
Subamolide E from Cinnamomum subavenium induces sub-G1 cell-cycle arrest and caspase-dependent apoptosis and reduces the migration ability of human melanoma cells.[Pubmed: 21707085]
The aim of this work was to investigate the anticancer cytotoxic effects of natural compound subamolide E on the human skin cancer melanoma A375.S2 cells. Subamolide E was isolated from Cinnamomum subavenium and demonstrated cytotoxicities in the cell-growth assay at concentration ranges from 0 to 100 μM at 24 h. Propidium iodide staining and flow cytometry analyses were used to evaluate cell-cycle distribution and found that subamolide E caused DNA damage in the sub-G1 phase with a dose-dependent manner after 24 h of treatment. According to the western blot result, subamolide-E-treated cells with the increase of caspase-dependent apoptotic proteins induced related pathway mechanisms. Subamolide E also showed antimigratory activities of A375.S2 cells on the wound-healing assay. Finally, subamolide E demonstrated minor cytotoxicities to normal human skin cells (keratinocytes, melanocytes, and fibroblasts); therefore, it is a potential chemotherapeutic agent against skin melanoma.
Isolinderanolide B, a butanolide extracted from the stems of Cinnamomum subavenium, inhibits proliferation of T24 human bladder cancer cells by blocking cell cycle progression and inducing apoptosis.[Pubmed: 21196431]
Isolinderanolide B (IOB), a butanolide extracted from the stems of Cinnamomum subavenium, was investigated for its antiproliferative activity in T24 human bladder cancer cells. To identity the anticancer mechanism of IOB, its effect on apoptosis, cell cycle distribution, and levels of p53, p21 Waf1/Cip1, Fas/APO-1 receptor, and Fas ligand was assayed. Enzyme-linked immunosorbent assay showed that the G0/G1 phase arrest is because of increase in the expression of p21 Waf1/Cip1. An enhancement in Fas/APO-1 and membrane-bound Fas ligand (mFasL) might be responsible for the apoptotic effect induced by IOB. This study reports the novel finding that the induction of p21 Waf1/Cip1 and activity of the Fas/mFas ligand apoptotic system may participate in the antiproliferative activity of IOB in T24 cells.
Identifying melanogenesis inhibitors from Cinnamomum subavenium with in vitro and in vivo screening systems by targeting the human tyrosinase.[Pubmed: 21054558]
Tyrosinase is known to be the first two and rate-limiting enzyme in the synthesis of melanin pigments responsible for colouring skin, hair and eyes. Tyrosinase inhibition is one major strategy used to treat hyperpigmentation. In human skin melanocytes, the cellular tyrosinase inhibition was examined by the conversion of l-tyrosine and oxidation of l-DOPA to dopaquinone. We evaluated the skin pigmentation inhibitor effects with both in vitro and in vivo systems to find skin-whitening agents without cytotoxic concerns. First, linderanolide B and subamolide A were isolated from the stems of Cinnamomum subavenium and exhibited mushroom tyrosinase inhibition. Then, these two herbal compounds were proved to have good pigmentation inhibitory abilities at low doses and demonstrated free cytotoxicities to normal human skin cells and zebrafish system. With molecular docking, in a virtual model of human tyrosinase, linderanolide B and subamolide A displayed meta(l) -coordinating interactions with Cu(2+) ions. The results obtained from biological assays showed that linderanolide B and subamolide A possessed anti-tyrosinase properties, which exhibited potential for application in medical cosmetology.


