Chloranthus multistachys
Chloranthus multistachys
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Chloranthus multistachys
- Cat.No. Product Name CAS Number COA
-
BCN5893
Rosmarinic acid20283-92-5
Instructions
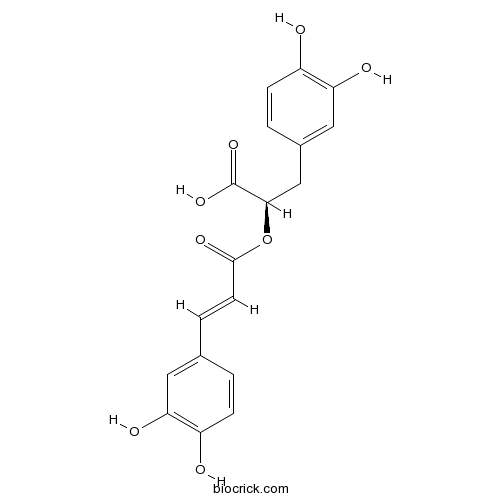
-
BCN2757
Ajugasterone C23044-80-6
Instructions
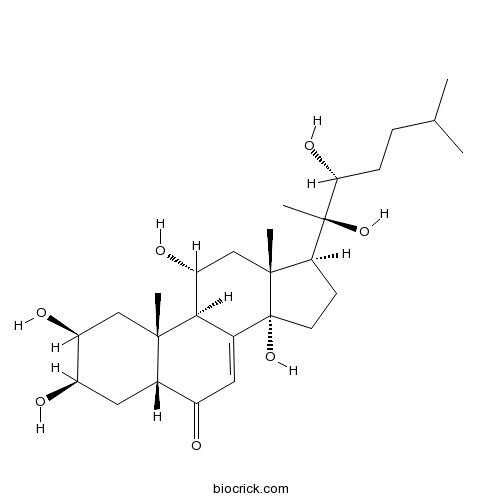
-
BCN5540
Bergenin477-90-7
Instructions
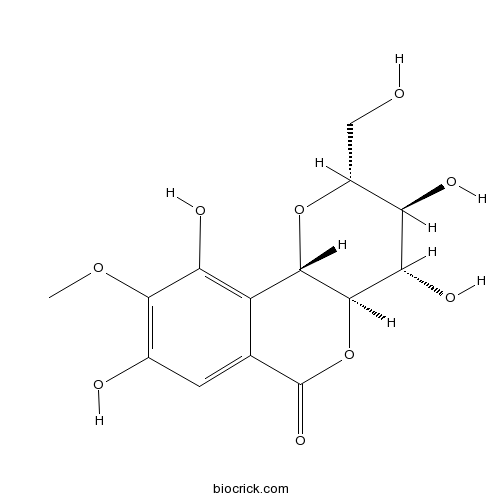
[Study on chemical constituents from Chloranthus multistachys].[Pubmed: 28901072]
To investigate the chemical constituents from the shoots of Chloranthus multistachys.All compounds wereisolated by using a combination of various chromatographic techniques including silica gel, ODS, Sephadex LH-20, reversed-phase HPLC, and other methods.Their structures were elucidated by the nuclear magnetic resonance (NMR), mass spectrometry, and other modernspectroscopies.As a result, 19 compounds were isolated from the shoots of C.multistachys and identified as zederoneepoxide(1), chlomultin C(2), curcolonol(3), sarcaglaboside A(4), zedoarofuran(5), (1E,4Z)-8-hydroxy-6-oxogermacra-1(10), 4,7(11)-trieno-12,8-lactone(6), chloranoside A(7), istanbulin A(8), (8α)-6,8-dihydroxycadina-7(11),10(15)-dien-12-oicacid-γ-lactone(9), codonolactone(10), lasianthuslactone A(11), 12,15-epoxy-5αH,9βH-labda-8(17),13-dien-19-oicacid(12), 12R,15-dihydroxylabda-8(17),13E-dien-19-oicacid(13), N-transcinnamoyltyramine(14), trans-N-p-coumaroyltyramine(15), dibutyl phthalate (16), flavokawain A(17), bergenin(18), and enedione(19).Compounds 1, 2, 4, 7-10, 12-19 were isolated from C.multistachys for the first time and compounds 14-19 were obtained from the genus Chloranthus for the first time.
Two new polyhydroxysterols produced by Fusarium solani, an endophytic fungus from Chloranthus multistachys.[Pubmed: 27000714]
A highly antagonistic endophytic fungus, designated strain CL39, was originated from the leaves of Chloranthus multistachys collected in Wulong of Chongqing municipality of China in November 2015. The strain was identified as Fusarium solani based on morphological characteristics, 5.8S gene and internal transcribed spacer sequence analysis. Two new compounds, 2β, 9α-dihydroxy-5α-methoxyergosta-7, 22-diene (1), 2β, 6β-dihydroxy-5α-methoxyergosta-7, 22-diene (2) have been isolated from the culture broth of the strain. Structures of the new compounds were elucidated by detailed analysis of their spectroscopic data aided by the comparison with reported data of related derivatives, and found to belong to the polyhydroxylated steroids with a hydroxyl at C-2 instead of C-3, a rare structure among the steroids. The extract of this strain and all isolated compounds were evaluated for their antagonistic activities.
In vitro inhibitory effects of terpenoids from Chloranthus multistachys on epithelial-mesenchymal transition via down-regulation of Runx2 activation in human breast cancer.[Pubmed: 25636886]
From Chloranthus multistachys, three terpenoids - lupeol (1), henrilabdane B (2), and istanbulin A (3) were isolated. Structures of compounds were established by NMR and MS. We reported here that ISTA (3) suppressed cell invasion, but lupeol (1) and henrilabdane B (2) did not. Furthermore, ISTA significantly inhibited the ability of adhesion and migration in vitro. Next, mechanisms of ISTA-induced inhibitory effects on in vitro metastasis were investigated. Sequential treatment data revealed that ISTA dramatically inhibited EGF-induced EMT. Western blot indicated that ISTA also significantly suppressed expression of E-cadherin, vimentin, and slug. In addition, ISTA inhibited Runx2 activation and phosph-Runx2 expression. Collectively, ISTA exhibited significant inhibitory effects on in vitro metastatic potential via inducing EMT inhibition, which may be associated with inhibition of transcriptional activity of Runx2.
An online coupled peritoneal macrophage/cell membrane chromatography and high-performance liquid chromatography/mass spectrometry method to screen for anti-inflammatory components from the Chinese traditional medicine Chloranthus multistachys Pei.[Pubmed: 23760986]
Cell membrane chromatography (CMC) is a chromatographic biological affinity method that uses specific cell membranes as the stationary phase. In this study, a novel peritoneal macrophage/cell membrane chromatography (PM/CMC)-online-high performance liquid chromatography/mass spectrometry (HPLC/MS) method was established to screen for the anti-inflammatory components from traditional Chinese medicines using hydrocortisone and dexamethasone as standards. The stationary phase of the CMC employed mouse peritoneal macrophage cell membranes. This method was applied to the purification and identification of components in extracts of Chloranthus multistachys Pei. The major component retained by CMC was identified as isofraxidin by HPLC/MS. In vitro experiments revealed that IF was able to inhibit the production of nitric oxide and tumor necrosis factor-α in lipopolysaccharide-stimulated mice and peritoneal macrophages in a dose-dependent manner. The results demonstrated that the PM/CMC-online-HPLC/MS is an effective screening system for the rapid detection, enrichment, and identification of target components from complex samples.
[Study on the chemical constituents of Chloranthus multistachys].[Pubmed: 23320357]
To isolate and identify the chemical constituents of the root of Chloranthus multistachys Pei.
Sesquiterpenoids from Chloranthus multistachys.[Pubmed: 23312365]
An 8,9-seco-lindenane disesquiterpenoid, chloramultiol G, four eudesmane sesquiterpenoids, ent-(3R)-3-hydroxyatractylenolide III and multistalactones A-C, and four guaiane sesquiterpenoids, (1R,4S,5R,8S,10S)-zedoalactone A and multistalactones D-F, along with 14 known compounds, were isolated from whole plant tissues of Chloranthus multistachys. Their structures were established by extensive NMR experiments in conjunction with mass spectrometry. Except for chloramultiol G, the absolute stereochemistries of the other eight were confirmed by single-crystal X-ray crystallography and CD spectra. Nine compounds were tested for cytotoxicity against five human tumor cell lines and for antifungal activity against four microorganisms in vitro, but all were inactive.
Chemical composition and antimicrobial activity of the essential oils from Chloranthus japonicus Sieb. and Chloranthus multistachys Pei.[Pubmed: 21319707]
We examined the composition and antimicrobial activity of two essential oils from Chloranthus japonicus Sieb. and Chloranthus multistachys Pei. GC-FID and GC-MS analyses identified 48 and 39 compounds, which represented 95.56% and 94.58%, respectively, of all components in these oils. Of these, 28 compounds were common to both, with a relatively high amount of oxygenated monoterpenes (50.95% and 39.97%). Antimicrobial properties were evaluated in vitro via disc diffusion and microbroth dilution assays. Activities were strong against most tested microorganisms, with inhibition zones ranging from 8.1 to 22.2 mm. For both species, minimum values for inhibitory and bactericidal concentrations were 0.39 to 12.50 mg/mL and 0.78 to 50.00 mg/mL, respectively. These results suggest that these essential oils are potent natural sources of antimicrobial agents for the medicinal and pharmaceutical industries.
Four sesquiterpenoids from Chloranthus multistachys.[Pubmed: 20552493]
Four new sesquiterpenoids, chlomultins A-D (1-4), were isolated from the whole plant of Chloranthus multistachys. Their structures were established on the basis of spectroscopic analysis.
Chloramultiols A-F, lindenane-type sesquiterpenoid dimers from Chloranthus multistachys.[Pubmed: 20392109]
Six new highly complex lindenane-type sesquiterpenoid dimers, chloramultiols A-F (1-6), along with six known analogues, were isolated from the whole plant of Chloranthus multistachys. The structures of 1-6 were elucidated on the basis of mass spectrometry (MS) and 1D and 2D NMR spectroscopic analysis. Among them, compounds 1 and 6 contain a unique 18-membered macrocyclic triester ring. All compounds isolated were evaluated for the inhibition of the growth of five tumor cell lines.


