Cerbera manghas
Cerbera manghas
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Cerbera manghas
- Cat.No. Product Name CAS Number COA
-
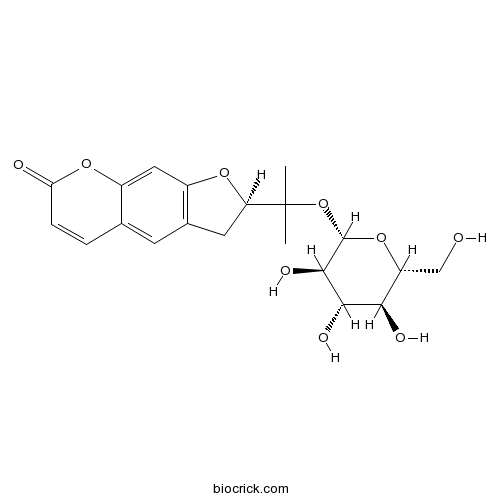
BCN2378
Nodakenin495-31-8
Instructions
Cerbera manghas methanol extract exerts anti-inflammatory activity by targeting c-Jun N-terminal kinase in the AP-1 pathway.[Pubmed: 27562319]
Cerbera manghas L. (Apocynaceae) is a medicinal plant traditionally used to ameliorate the clinical signs of inflammatory diseases and hypertension.
Polyketides with α-Glucosidase Inhibitory Activity from a Mangrove Endophytic Fungus, Penicillium sp. HN29-3B1.[Pubmed: 26230970]
Five new compounds, pinazaphilones A and B (1, 2), two phenolic compounds (4, 5), and penicidone D (6), together with the known Sch 1385568 (3), (±)-penifupyrone (7), 3-O-methylfunicone (8), 5-methylbenzene-1,3-diol (9), and 2,4-dihydroxy-6-methylbenzoic acid (10) were obtained from the culture of the endophytic fungus Penicillium sp. HN29-3B1, which was isolated from a fresh branch of the mangrove plant Cerbera manghas collected from the South China Sea. Their structures were determined by analysis of 1D and 2D NMR and mass spectroscopic data. Structures of compounds 4 and 7 were further confirmed by a single-crystal X-ray diffraction experiment using Cu Kα radiation. The absolute configurations of compounds 1-3 were assigned by quantum chemical calculations of the electronic circular dichroic spectra. Compounds 2, 3, 5, and 7 inhibited α-glucosidase with IC50 values of 28.0, 16.6, 2.2, and 14.4 μM, respectively, and are thus more potent than the positive control, acarbose.
Acaricidal activity against Panonychus citri and active ingredient of the mangrove plant Cerbera manghas.[Pubmed: 25918788]
Cerbera manghas is a mangrove plant which possesses comprehensive biological activities. A great deal of research has been undertaken on the chemical constituents and medical functions of C. manghas; insecticidal and antifungal activities have also been reported, but the acaricidal activity has not been studied. In our study, the acaricidal activity and active substances of C. manghas were investigated using a spray method, which showed that the methanol extracts of the fruit, twigs and leaves exhibited contact activity against female adults of Panonychus citri, with LC50 values at 24 h of 3.39 g L(-1), 4.09 g L(-1) and 4.11 g L(-1), respectively. An acaricidal compound was isolated from C. manghas by an activity-guided isolation method, and identified as (-)-17β-neriifolin, which is a cardiac glycoside. (-)-17β-Neriifolin revealed high contact activity against female adults, nymphae, larvae and eggs of P. citri, with LC50 values at 24 h of 0.28 g L(-1), 0.29 g L(-1), 0.28 g L(-1) and 1.45 g L(-1), respectively.
Vermistatin derivatives with α-glucosidase inhibitory activity from the mangrove endophytic fungus Penicillium sp. HN29-3B1.[Pubmed: 25116120]
Three new vermistatin derivatives, 6-demethylpenisimplicissin (1), 5'-hydroxypenisimplicissin (2), and 2''-epihydroxydihydrovermistatin (3), along with five known vermistatin analogues, methoxyvermistatin (4), vermistatin (5), 6-demethylvermistatin (6), hydroxyvermistatin (7), and penisimplicissin (8), were isolated from the culture of the mangrove endophytic fungus Penicillium sp. HN29-3B1 from Cerbera manghas. Their structures were elucidated mainly by nuclear magnetic resonance spectroscopy. The absolute configurations of compounds 1 and 2 were deduced on the basis of circular dichroism data. The absolute structures of compounds 3 and 5 were confirmed by a single-crystal X-ray diffraction experiment using Cu Kα radiation. In the bioactivity assay, compounds 1 and 3 exhibited α-glucosidase inhibitory activity with IC50 values of 9.5 ± 1.2 and 8.0 ± 1.5 µM, respectively. The plausible biosynthetic pathways for all compounds are discussed.
The principal toxic glycosidic steroids in Cerbera manghas L. seeds: identification of cerberin, neriifolin, tanghinin and deacetyltanghinin by UHPLC-HRMS/MS, quantification by UHPLC-PDA-MS.[Pubmed: 24878878]
The toxicity of the sea mango (Cerbera manghas L.) is well known. The plant is ranked as one of the deadliest of the southern Asian coastline. Cardenolidic heterosides are responsible for the cardiotoxicity of trees of the Cerbera genus. We have identified and determined the concentration of the principal glycosidic steroids present in the seeds of sea mangos (Thailand). Drug screening of an extract of the seeds was carried out using ultra-high performance liquid chromatography coupled to photodiode array detection and mass spectrometry (UHPLC-PDA-MS) with quantification at 219nm. Identification was confirmed by UHPLC-HRMS. Deacetyltanghinin (m/z 549.3055±2ppm), neriifolin (m/z 535.3259±2ppm), tanghinin (m/z 591.3169±2ppm) and cerberin (577.3375±2ppm) were the most abundant glycosidic steroids present in the sea mango seeds. A seed of the dried ripe fruit had concentrations of 1209.1, 804.2, 621.4 and 285.9μg/g, respectively. A seed of the fresh unripe fruit had concentrations of 49.4, 47.0, 3.5 and 2.3μg/g.
Syk and Src are major pharmacological targets of a Cerbera manghas methanol extract with kaempferol-based anti-inflammatory activity.[Pubmed: 24342777]
Cerbera manghas L. (Apocynaceae), a semi-mangrove medicinal plant distributed throughout tropical and subtropical countries, is traditionally known to possess analgesic, anti-inflammatory, anti-convulsant, cardiotonic, and hypotensive activity. In vitro and in vivo anti-inflammatory activities of a methanol extract of the leaves of Cerbera manghas and the underlying molecular mechanisms were investigated to validate the ethnopharmacological use of this plant.
Marine natural product des-O-methyllasiodiplodin effectively lowers the blood glucose level in db/db mice via ameliorating inflammation.[Pubmed: 23852084]
des-O-methyllasiodiplodin (DML) from Cerbera manghas has shown antagonistic activity against mineralocorticoid receptor (MR). Considering the involvement of MR in the insulin tolerance, we attempted to investigate the potential of DML in the treatment of type 2 diabetes mellitus (T2DM).
Tanghinigenin from seeds of Cerbera manghas L. induces apoptosis in human promyelocytic leukemia HL-60 cells.[Pubmed: 21787626]
Tanghinigenin, a cardiac glycoside, is isolated from the seeds of Cerbera manghas L. In this study, we demonstrated that tanghinigenin reduced the viability of human promyelocytic leukemia HL-60 cells in a time- and dose-dependent manner, and efficiently induced apoptosis in HL-60 cells as evidenced by the Annexin V/PI binding assay, DNA fragmentation and AO/EB staining studies. In addition, stimulation of HL-60 cells with tanghinigenin induced a series of intracellular events including the activation of caspase-3, -8, and -9, as well as up-regulation of Fas and FasL protein level. Taken together, caspase activation and Fas/FasL interaction was found to be involved in tanghinigenin-induced HL-60 cell apoptosis.


