Caragana changduensis
Caragana changduensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Caragana changduensis
- Cat.No. Product Name CAS Number COA
-
BCN5946
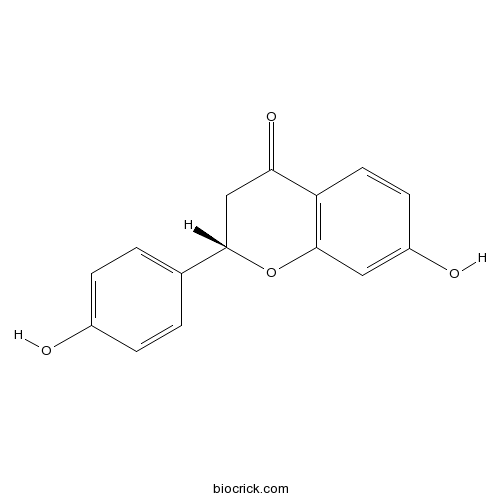
Liquiritigenin578-86-9
Instructions
[Chemical Constituents from Caragana changduensis].[Pubmed: 30079714]
To study the chemical constituents of the red heartwood of the stems and roots of Caragana changduensis.
[Isoflavonoids from Caragana changduensis and their nitric oxideinhibitory activities].[Pubmed: 26790296]
Ten isoflavonoids were isolated from the heartwoods of Caragana changduensis Lion f. by means of various column chromatographic techniques. Based on the detailed spectral data analysis (MS and NMR), as well as comparison with the literatures, their chemical structures were determined as 7,2'-dihydroxy-8,4'-dimethoxyisoflavone (1), 4'-hydroxy-7,3'-dimethoxyisoflavone (2), 5, 7, 4'-trihydroxy-2',5'-dimethoxyisoflavone (3), prunetin (4), afrormosin (5), odoratin (6), genistein (7), texasin (8), pratensein (9), and 6,7,3'-trihydroxy-4'-methoxyisoflavone (10). Among them, compounds 1-3 and 9-10 were isolated from the Caragana genus for the first time. All the compounds were obtained from this species for the first time. In the preliminary assays, compounds 1, 2, 6, and 7 possessed significant inhibitory effects on NO production, with IC50 values of 48.12, 25.32, 62.71, 43.59 μmol x L(-1), respectively.


