Campylotropis hirtella
Campylotropis hirtella
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Campylotropis hirtella
- Cat.No. Product Name CAS Number COA
-
BCN6314
Procyanidin B120315-25-7
Instructions
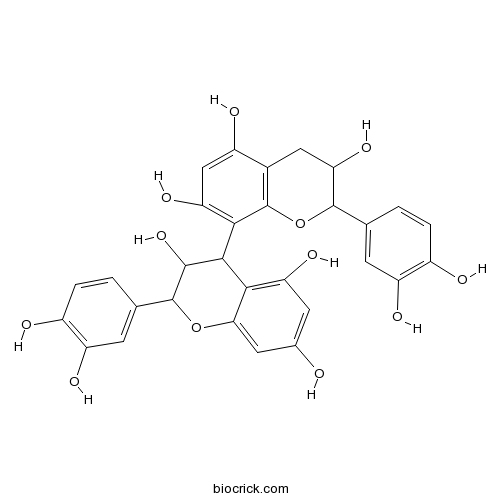
-
BCN6315
Procyanidin B229106-49-8
Instructions
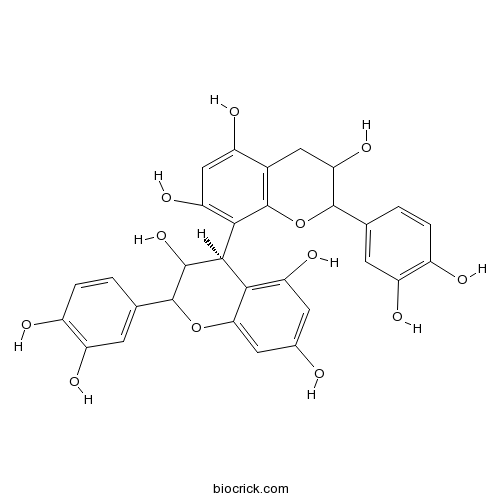
Human neutrophil elastase inhibitory potential of flavonoids from Campylotropis hirtella and their kinetics.[Pubmed: 27558014]
None
Chemical Constituents from Campylotropis hirtella.[Pubmed: 27220079]
A phytochemical investigation on the roots of Campylotropis hirtella afforded nine new isoflavones (3-9, 12, 15), two new isoflavans (10 and 11), one new coumestan (1), and three new prenylated benzoic acid derivatives (2, 13, 14), together with twenty-four known compounds. Their structures were established by spectroscopic analysis and circular dichroism data. The isolated compounds were also evaluated for their antibacterial activities against Enterococcus faecalis, Salmonella gallinarum, Streptococcus suis, Streptococcus agalactiae, Aeromonas hydrophila, Pseudomonas aeruginosa, Bacillus subtilis, Riemerella anatipestifer, and Vibrio alginolyticus.
Highly potent tyrosinase inhibitor, neorauflavane from Campylotropis hirtella and inhibitory mechanism with molecular docking.[Pubmed: 26706112]
Tyrosinase inhibition may be a means to alleviate not only skin hyperpigmentation but also neurodegeneration associated with Parkinson's disease. In the course of metabolite analysis from tyrosinase inhibitory methanol extract (80% inhibition at 20 μg/ml) of Campylotropis hirtella, we isolated fourteen phenolic compounds, among which neorauflavane 3 emerged as a lead structure for tyrosinase inhibition. Neorauflavane 3 inhibited tyrosinase monophenolase activity with an IC50 of 30 nM. Thus this compound is 400-fold more active than kojic acid. It also inhibited diphenolase (IC50=500 nM), significantly. Another potent inhibitor 1 (IC50=2.9 μM) was found to be the most abundant metabolite in C. hirtella. In kinetic studies, compounds 3 showed competitive inhibitory behavior against both monophenolase and diphenolase. It manifested simple reversible slow-binding inhibition against monophenolase with the following kinetic parameters: Ki(app)=1.48 nM, k3=0.0033 nM(-1) min(-1) and k4=0.0049 min(-1). Neorauflavane 3 efficiently reduced melanin content in B16 melanoma cells with 12.95 μM of IC50. To develop a pharmacophore model, we explored the binding mode of neuroflavane 3 in the active site of tyrosinase. Docking results show that resorcinol motif of B-ring and methoxy group in A-ring play crucial roles in the binding the enzyme.
A new potent immunosuppressive isoflavanonol from Campylotropis hirtella.[Pubmed: 26221996]
Four new flavonoids were isolated from Campylotropis hirtella and these are a chromone and a 2H-chromene, an isoflavone and an isoflavanonol. The structures of these compounds were elucidated by extensive spectroscopic measurements. All of the compounds were assessed for immunosuppressive activity. Compound 4 showed very strong T lymphocyte suppression activity (IC50: 0.13 μM) and potent B lymphocyte suppression activity (IC50: 0.26 μM). Due to its potent immunosuppressive activity and lower cytotoxicity, further structure-activity studies will be pursued on this compound.
New flavonoids from Campylotropis hirtella with immunosuppressive activity.[Pubmed: 24709073]
In an effort to identify natural compounds with immunosuppressive activity, nine new flavonoids, including one isoflav-3-ene derivative (1), one coumaronochromone (2), two isoflavanones (3, 4), one isoflavone derivative (6), one isoflavone (7), three flavonols (8, 9, 10), as well as one known compound, hydroisoflavone C (5), were isolated from the roots of Campylotropis hirtella. The structures of these compounds were elucidated by extensive spectroscopic measurements. All of the compounds were assessed for immunosuppressive activity. Among the isolates, compound 2 showed good inhibitory activity against mitogen-induced splenocyte proliferation with an IC50 of 0.28 μM and relatively low cytotoxicity.
Flavonoids from the roots of Campylotropis hirtella.[Pubmed: 21678233]
Eight new flavonoids, including three pterocarpene derivatives, hirtellanines C-E, two flavanones, hirtellanines F and G, three isoflavanones, hirtellanines H-J, as well as four known compounds were isolated from the roots of Campylotropis hirtella. The structures of the new compounds were elucidated by spectroscopic analyses, including 2D-NMR techniques. Pharmacological investigation indicated that the new compounds, particularly hirtellanines D and G, possessed potent immunosuppressive activities and low relative cytotoxicities.
[Determination of two bio-active compounds in Campylotropis hirtella (Franch.) Schindl. using reversed-phase high performance liquid chromatography].[Pubmed: 21438367]
A method of reversed-phase high performance liquid chromatography (RP-HPLC) using diode array detection (DAD) was developed for the quantitative determination of 3'-geranyl-5,7,4'-trihydroxyisoflavone (compound 1) and 8,9-dihydroxy-1-methoxy-[6',6'-dimethylpyrano(2',3': 2,3)]pterocarpene (compound 2) in Campylotropis hirtella. The separation and quantification were achieved using an Agilent Zorbax SB-C18 column (250 mm x 4.6 mm, 5 microm), and mobile phases of acetonitrile and 0. 1% formic acid with gradient elution at a flow rate of 1.0 mL/min and 30 degrees C. The calibration curves for compounds 1 and 2 were linear in the ranges of 4.4-13.2 microg and 0.428-1.284 microg, respectively. The recoveries were 99.65% and 99.11% with the relative standard deviations of 1.83% and 2.59% (n=5), respectively. This RP-HPLC-DAD method is rather simple, accurate and convenient. It can be used for the quantitative determination of the active flavonoids in Campylotropis hirtella (Franch.) Schindl.
Isoflavonoids from the roots of Campylotropis hirtella.[Pubmed: 20013635]
From the dried roots of Campylotropis hirtella (Franch.) Schindl., five novel isoflavonoids, 3( S)-7,2',4'-trihydroxy-5,5'-dimethoxy-6-(3-methylbut-2-enyl)-isoflavan ( 1), 3( S)-2',4'-dihydroxy-5,5'-dimethoxy-(6'',6''-dimethylpyrano)-(2'',3'':7,6)-isoflavan ( 2), 3( R)-5,4'-dihydroxy-2'-methoxy-3'-(3-methylbut-2-enyl)-(6'',6''-dimethylpyrano)-(7,6 : 2'',3'')-isoflavanone ( 3), 3( R)-5,4'-dihydroxy-2'-methoxy-(6'',6''-dimethylpyrano)-(7,6: 2'',3'')-isoflavanone ( 4), and 3( R)-5,4'-dihydroxy-7,2'-dimethoxy-6-geranylisoflavanone ( 5) were isolated. The structures of the compounds were elucidated on the basis of spectroscopic analysis. All isolates exhibited good immunosuppressive activities on mitogen-induced splenocyte proliferation, and their cytotoxicity on splenic lymphocytes was also tested.
Geranylated flavonoids from the roots of Campylotropis hirtella and their immunosuppressive activities.[Pubmed: 19572647]
In an effort to identify new immunosuppressive agents from natural sources, 12 new geranylated flavonoids, 5,7,4'-trihydroxy-3'-[7-hydroxy-3,7-dimethyl-2(E)-octenyl]isoflavone (1), a racemate of 5,7,2',4'-tetrahydroxy-3'-[7-hydroxy-3,7-dimethyl-2(E)-octenyl]isoflavanone (2), 2''(S)-5,7-dihydroxy-[2''-methyl-2''-(4-methyl-3-pentenyl)pyrano]-5'',6'':3',4'-isoflavone (3), (2''S,3''R,4''S)-5,7,3'',4''-tetrahydroxy[2''-methyl-2''-(4-methyl-3-pentenyl)pyrano]-5'',6'':3',4'-isoflavone (4), a racemate of 3'-geranyl-5,7,2',4'-tetrahydroxyisoflavanone (5), a racemate of 3'-geranyl-4'-methoxy-5,7,2'-trihydroxyisoflavanone (6), 3'-geranyl-5,7,4',5'-tetrahydroxyisoflavone (8), 3'-geranyl-5,7,2',5'-tetrahydroxyisoflavone (9), 3'-geranyl-4'-methoxy-5,7,2'-trihydroxyisoflavone (10), 2(R),3(R)-3'-geranyl-2,3-trans-5,7,4'-trihydroxyflavonol (12), (2R,3R)-6-methyl-3'-geranyl-2,3-trans-5,7,4'-trihydroxyflavonol (13), and 5,7-dihydroxy-4'-O-geranylisoflavone (14), were isolated from the roots of Campylotropis hirtella (Franch.) Schindl. together with three previously described flavonoids. Their structures were elucidated by spectroscopic measurements, including two-dimensional nuclear magnetic resonance (NMR) techniques. The immunosuppressive effects of these compounds were assessed using mitogen-induced splenocyte proliferation, and the cytotoxicity of the compounds was also examined. The IC50 values of the compounds were found to be in the range of 1.49-61.23 microM for T lymphocyte suppression and 1.16-73.07 microM for B lymphocyte suppression. An analysis of their structure-activity relationships revealed that an isoflavonoid carbon skeleton with a C10 substituent at the C3' position was necessary for the activity. As many of the compounds exhibited good immunosuppressive activities, they may be promising as novel immunosuppressive agents.
Hirtellanines A and B, a pair of isomeric isoflavonoid derivatives from Campylotropis hirtella and their immunosuppressive activities.[Pubmed: 19481938]
A pair of isomeric isoflavonoid derivatives, Hirtellanines A (1) and B (2), has been isolated from the roots of Campylotropis hirtella (Franch.) Schindl. and their structures were elucidated on the basis of spectroscopic methods, with special emphasis on 1D and 2D NMR techniques. The in vitro assay showed that Hirtellanine A had strong B lymphocyte suppression activity (IC(50): 0.06microM) and T lymphocyte suppression activity (IC(50): 0.92microM). Hirtellanine B showed moderate B lymphocyte suppression activity (IC(50): 3.00microM) and T lymphocyte suppression activity (IC(50): 9.55microM). Due to the potent immunosuppressive activities and lower cytotoxicity, Hirtellanine A could be a promising lead towards novel immunosuppressive agents.


