Balanophora japonica
Balanophora japonica
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Balanophora japonica
- Cat.No. Product Name CAS Number COA
-
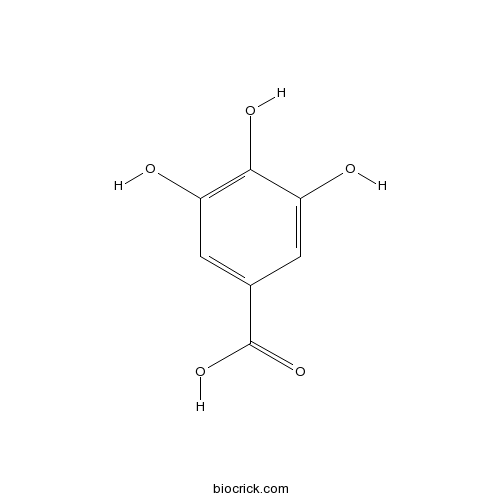
BCN1668
Gallic acid149-91-7
Instructions
Morphology and phylogenetics of two holoparasitic plants, Balanophora japonica and Balanophora yakushimensis (Balanophoraceae), and their hosts in Taiwan and Japan.[Pubmed: 21894574]
Balanophora japonica and B. yakushimensis are two putatively agamospermic taxa previously reported from southern Japan. Their inflorescences superficially represent those of B. laxiflora and B. fungosa. In this study we confirmed their presence in Taiwan by morphological and phylogenetic analysis using nuclear 18S rDNA and nrITS sequences with related taxa. B. japonica, B. yakushimensis, and B. laxiflora formed a well-supported clade that is distinct from other Balanophora. All three taxa also show considerable differences on morphological and nucleotide sequence differences, therefore the name of B. yakushimensis is retained. The results provide new insights on the intrageneric classification of Balanophora and suggest the positioning of female flowers should be down-weighted. We also successfully identify the hosts of B. japonica and B. yakushimensis by amplifying chloroplast matK sequences from the connected root tissues. The results showed that B. japonica parasitizes on Symplocos species, and that B. yakushimensis parasitizes on Distylium racemosum in Japan and Schima superba in Taiwan's population.
New phenylpropanoids and in vitro α-glucosidase inhibitors from Balanophora japonica.[Pubmed: 20979022]
Five new phenylpropanoids, named balajaponins A-E (1-5), were isolated from Balanophora japonica, along with 24 known compounds. Among them, three hydrolysable tannins (6-8) showed specific in vitro α-glucosidase inhibition, with IC₅₀ values in the range of 1-4 µM. Kinetic analysis revealed that they all acted in a noncompetitive mode.
[Study of the mechanism of caffeoyl glucopyranoses in inhibiting HIV-1 entry using pseudotyped virus system].[Pubmed: 20423834]
To investigate the inhibitory activities of caffeoyl glucopyranoses purified from Balanophora japonica Makino on HIV entry and their mechanism.
[1,2,6-tri-O-galloyl-beta-D-glucopyranose inhibits gp41-mediated HIV envelope fusion with target cell membrane].[Pubmed: 18676243]
To observe the inhibitory effect of 1,2,6-Tri-O-galloyl-beta-D-glucopyranose (TGGP) from Balanophora japonica Makino on human immunodeficiency virus (HIV) entry into the host cells and explore the mechanisms.
Cytotoxic hydrolyzable tannins from Balanophora japonica.[Pubmed: 18302336]
Four hydrolyzable tannins named balanophotannins D-G ( 1- 4) were isolated from the aerial parts of the parasitic plant Balanophora japonica. Their structures were characterized on the basis of spectroscopic and chemical evidence. Balanophotannins D-G contain an oxidized hexahydroxydiphenoyl (HHDP) group. The absolute configurations of balanophotannins D ( 1) and F ( 3) were determined via the PGME method. Balanophotannin E ( 2) showed cytotoxicity to Hep G2 cancer cells with an IC 50 value of 4.22 microM.
Caffeoyl, coumaroyl, galloyl, and hexahydroxydiphenoyl glucoses from Balanophora japonica.[Pubmed: 11456097]
Eighteen new and sixteen known acyl glucoses having caffeoyl, coumaroyl, galloyl, and hexahydroxydiphenoyl groups were isolated from a medicinal parasitic plant, Balanophora japonica. Their structures were determined by spectroscopic and chemical methods. Caffeoyl ellagitannins, which have been rarely found in nature, were major phenolic constituents of this plant, and this is the first report of the isolation of ellagitannins from Balanophoraceae.


