Arundina graminifolia
Arundina graminifolia
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Arundina graminifolia
- Cat.No. Product Name CAS Number COA
-
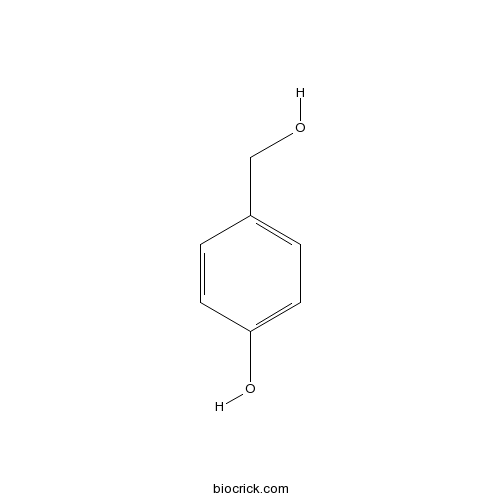
BCN4154
4-Hydroxybenzyl alcohol623-05-2
Instructions
Total Synthesis of Gramistilbenoids A, B, and C.[Pubmed: 29613790]
Stilbenes are biologically active metabolites of plants that have the potential to attenuate a broad range of human diseases. Gramistilbenoids are a class of natural products with a stilbene skeleton, isolated from the bamboo orchid ( Arundina graminifolia), and with significant cytotoxicity against cancer cell lines (NB4, A549, SHSY5Y, PC3, and MCF7). These are the first identified naturally occurring diphenylethylenes to possess a hydroxyethyl unit. However, some of these compounds are not abundant in nature, and thus, their synthesis is advantageous. This paper reports the first synthesis of gramistilbenoids A (1), B (2), and C (3), with overall yields of 10, 2, and 8% respectively. These natural products were synthesized using key reactions, such as Horner-Wadsworth-Emmons olefination, Stille coupling, and hydroboration-oxidation.
Arundinosides A-G, new glucosyloxybenzyl 2R-benzylmalate derivatives from the aerial parts of Arundina graminifolia.[Pubmed: 29170123]
Seven new glucosyloxybenzyl 2R-benzylmalate derivatives, arundinosides A-G (1-7) were isolated from the aerial parts of the bamboo orchid Arundina graminifolia. This is the first occurrence of this class of compounds in the genus Arundina. Their planar structures and absolute configuration were determined by extensive NMR spectroscopic data as well as chemical conversion. Their neuroprotective properties were also evaluated on their potential ability to reduce the beta amyloid damage on PC12 cell model.
Phenanthrenes from Arundina graminifolia and in vitro evaluation of their antibacterial and anti-haemolytic properties.[Pubmed: 28553728]
Chemical investigation and activity test of Arundina graminifolia led to the isolation of six phenanthrenes: blestriarene A (1), shancidin (2), densiflorol B (3), ephemeranthoquinone (4), coelonin (5) and lusianthridin (6). The isolated compounds demonstrated antibacterial and anti-haemolytic activities. It was found that compounds 1 and 2 had medium antibacterial activity against Staphylococcus aureus, Bacillus subtilis and Escherichia coli, with MICs of 20-40 μg/mL and MBCs of 40-320 μg/mL. Bactericidal mechanisms were explored. Rupture of cell wall and membrane and leakage of nuclear mass were observed by transmission electron microscopy (TEM). Moreover, compounds 1-3 attenuated the erythrocyte damage. Compounds 1 and 2 showed significant anti-haemolytic activity with inhibition rate about 50% at 16 μg/mL.
Two New Stilbenoids from the Aerial Parts of Arundina graminifolia (Orchidaceae).[Pubmed: 27801800]
None
Cryopreservation on a cryo-plate of Arundina graminifolia protocorms, dehydrated with silica gel and drying beads.[Pubmed: 27224527]
There are various methods for the cryopreservation of plant material, with each biological specimen potentially requiring protocol optimization to maximize success.
Arundina graminifolia var. revoluta (Arethuseae, Orchidaceae) has fern-type rheophyte characteristics in the leaves.[Pubmed: 25502073]
Morphological and molecular variation between Arundina graminifolia var. graminifolia and the dwarf variety, A. graminifolia var. revoluta, was examined to assess the validity of their taxonomic characteristics and genetic background for identification. Morphological analysis in combination with field observations indicated that A. graminifolia var. revoluta is a rheophyte form of A. graminifolia characterized by narrow leaves, whereas the other morphological characteristics described for A. graminifolia var. revoluta, such as smaller flowers and short stems, were not always accompanied by the narrower leaf phenotype. Molecular analysis based on matK sequences indicated that only partial differentiation has occurred between A. graminifolia var. graminifolia and A. graminifolia var. revoluta. Therefore, we should consider the rheophyte form an ecotype rather than a variety. Anatomical observations of the leaves revealed that the rheophyte form of A. graminifolia possessed characteristics of the rheophytes of both ferns and angiosperms, such as narrower palisade tissue cells and thinner spongy tissue cells, as well as fewer cells in the leaf-width direction and fewer mesophyll cell layers.
Cytotoxic deoxybenzoins and diphenylethylenes from Arundina graminifolia.[Pubmed: 24063582]
Eight new C-4-alkylated deoxybenzoins (1-8), three new diphenylethylenes (9-11), and five known diphenylethylenes were isolated from Arundina graminifolia. The structures of 1-11 were elucidated by spectroscopic methods including extensive 1D and 2D NMR techniques. Compounds 9-11 are the first naturally occurring diphenylethylenes possessing a hydroxyethyl unit. Compounds 1-11 were evaluated for cytotoxicity against five human tumor cell lines. Compounds 4, 5, and 9-11 showed significant cytotoxicity against five cancer cell lines, with IC50 values ranging from 1.8 to 8.7 μM.
Pollination and floral ecology of Arundina graminifolia (Orchidaceae) at the northern border of the species' natural distribution.[Pubmed: 23917792]
Arundina graminifolia is an early successional plant on Iriomote Island, the Ryukyus, Japan, where it is endangered. Populations flower for more than half a year, and many inflorescences bloom for one to several months. The nectarless gullet flowers, which open for up to six days, are self-compatible but cannot self-pollinate spontaneously; thus they rely on pollinating agents for capsule production. Field observations at two habitats identified at least six species of bees and wasps, primarily mate-seeking males of Megachile yaeyamaensis and Thyreus takaonis, as legitimate pollinators. Thus, this orchid is a pollinator generalist, probably owing to its long blooming period and simple flower morphology. Carpenter bees, which were previously reported to pollinate this orchid, frequently visited flowers but were too large to crawl into the labellum chamber and never pollinated the flowers. Extrafloral nectaries on inflorescences attracted approximately 40 insect taxa but were not involved with pollination. Fruit-set ratios at the population level varied spatiotemporally but were generally low (5.2-12.4 %), presumably owing to infrequent flower visits by mate-seeking pollinators and the lack of food rewards to pollinators.
[Antitumoral bibenzyl derivatives from tuber of Arundina graminifolia].[Pubmed: 22741464]
To isolate the bibenzyl derivatives from the tuber of Arundina graminifolia and evaluate the anti-tumor activity of these compounds in vitro.
Rapid floral senescence following male function and breeding systems of some tropical orchids.[Pubmed: 21972891]
No comparative study of floral senescence following male function among a range of tropical orchid genera has previously been undertaken. The timing and pattern of floral senescence and occurrence of fruit formation were studied following self-, geitonogamous and cross-pollination in 14 epiphytic and two terrestrial orchid species to determine their breeding system and assess the occurrence of floral abscission following pollinaria removal. Both pollination and pollinaria removal caused rapid floral senescence, and the pattern and timing of the floral changes were the same in all treatments. Six Dendrobium species and Pelatantheria insectifera were self-incompatible (SI) and eight other species, including one terrestrial species, were self-compatible (SC). Capsules produced from outcrossing in four SC species, Phalaenopsis cornu-cervi, Eria pubescens, Cleisostoma appendiculatum and Arundina graminifolia, were larger and heavier than those produced after selfing. Reductions in flower life span following pollinaria removal were positively correlated with flower size and longevity of unpollinated flowers but not with position in the inflorescence or nature of the breeding system. Rapid flower senescence following pollinaria removal reported here suggests that it may be widespread in tropical species. The significant association of the response with size of flowers and inflorescences among the species studied suggests that the cost of flower maintenance outweighs the benefit of remaining open for female function after pollinaria have been removed. Both SC and SI species were found among tropical orchids, but variation in capsule size following self- and cross-pollination indicates that there may be a reduction in seed production following selfing, even in SC species, and that fruit formation alone should not be taken as reliable evidence of full self-compatibility.


