Artemisia rupestris
Artemisia rupestris
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Artemisia rupestris
- Cat.No. Product Name CAS Number COA
-
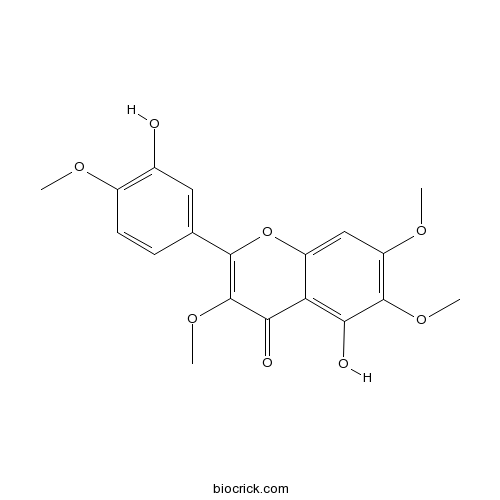
BCN5020
Vitexicarpin479-91-4
Instructions
-
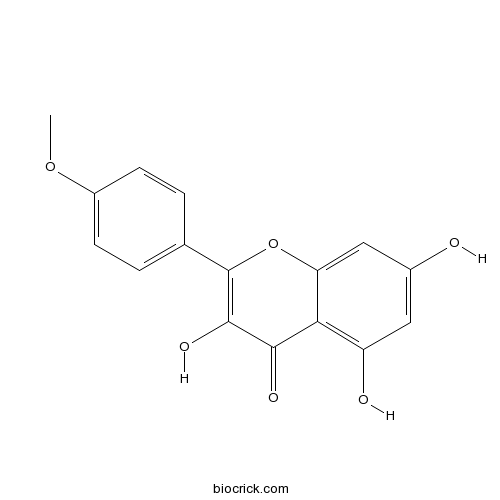
BCN5598
Kaempferide491-54-3
Instructions
-
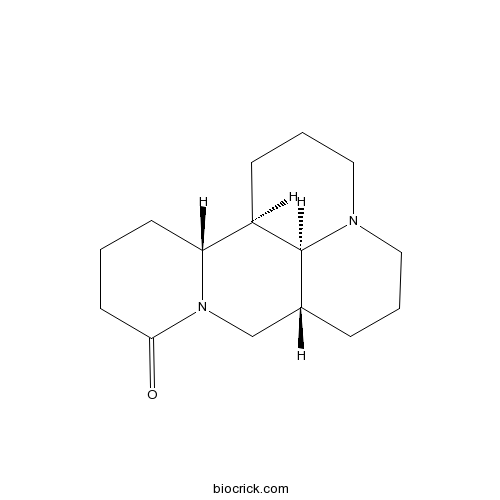
BCN4249
Sophoridine6882-68-4
Instructions
[Chemical constituents, biological activities and clinical applications of artemisia rupestris].[Pubmed: 29376253]
Artemisia rupestris is a traditional medicine in Uygur and Kazak in Xinjiang Province, mainly distributed in the territory of Xinjiang Altai area, Tianshan mountains and the Kunlun mountains, growing at an altitude of 1 500 to 4 000 meters of grassland and forest areas. As the broad research on chemical constituents, pharmacological activity, the effective components of A. rupestris have attracted the interest to make up new drugs. Based on the latest research from A. rupestris, identification and geographic distribution, chemical constituents, pharmacological effects, clinical applications were summarized in this article, in the view of Medicinal Ethnobotany. At the same time, some suggestions were proposed for future research.
Cytotoxic, anti-cancer, and anti-microbial effects of different extracts obtained from Artemisia rupestris.[Pubmed: 28569514]
Artemisia rupestris is a part of traditional Kazakh folk medicine. Extracts obtained from this plant are used to treat various diseases, including cancer. This study evaluates the anti-microbial, cytotoxic, and anti-cancer effects of different extracts of the plant. Different extraction techniques were used and the resultant activities were compared. Extracts of A. rupestris were prepared from the flowers plus the leaves and from the stems. The antimicrobial activity against Candida albicans and Staphylococcus aureus was quantified. Cell lines L1210 and THP-1 were used to evaluate the cytotoxic potential of these extracts in vitro. The anti-cancer effect was tested using L1210-induced tumorgenesis in mouse model. The aqueous extract of stems was the most active against C. albicans, whereas the methanolic extract of flowers plus leaves especially inhibited the growth of S. aureus. The aqueous extracts were found to be non-cytotoxic for both cell lines, whereas the lipophilic extracts showed cytotoxic effects. The extract obtained from flowers plus leaves was more cytotoxic than that from stems. The tested extracts showed no anti-cancer potential. The results obtained testify to the relatively safe consumption of aqueous extracts of A. rupestris, but lipophilic extracts showed toxic effects and their consumption should be considered more carefully.Key words: L1210 cell line THP-1 cell line microwave-assisted extraction ultrasonic-assisted extraction Candida albicans Staphylococcus aureus.
Adjuvant-active aqueous extracts from Artemisia rupestris L. improve immune responses through TLR4 signaling pathway.[Pubmed: 28111143]
None
Nitric Oxide Inhibitory Dimeric Sesquiterpenoids from Artemisia rupestris.[Pubmed: 26696523]
Twelve new dimeric sesquiterpenoids (1-12) were isolated from the dried whole plants of Artemisia rupestris. Their structures were determined using MS and NMR data, and the absolute configurations were elucidated on the basis of experimental and calculated ECD spectra. Compounds 1-9 are presumably formed via biocatalyzed [2+2] or [4+2] cycloaddition reactions. Stereoselectivity of the [4+2] Diels-Alder reaction dictated the formation of endo-products. The dimeric sesquiterpenoids exhibited moderate inhibition on NO production stimulated by lipopolysaccharide in BV-2 microglial cells, with IC50 values in the range 17.0-71.8 μM.


