Artemisia myriantha
Artemisia myriantha
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Artemisia myriantha
- Cat.No. Product Name CAS Number COA
-
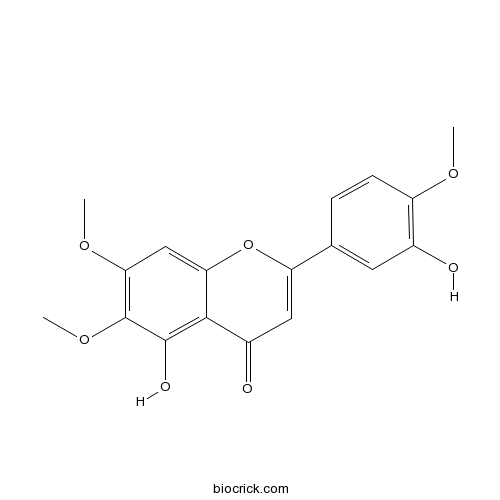
BCN4405
Eupatorin855-96-9
Instructions
[Sesquiterpenoids from aerial parts of Artemisia myriantha].[Pubmed: 28914025]
The present study is to investigate the chemical constituents from the aerial parts of Artemisia myriantha. The chemical constituents were isolated by column chromatographies over silica gel, Sephadex LH-20, ODS and Semi-prep HPLC, and the structures were identified by NMR and MS data. Thirteen known compounds were isolated and identified as: blumenol A (1),(+)-dehydrovomifoliol (2),(+)-3-hydroxy-β-ionone (3),(3R, 6R, 7E)-3-hydroxy-4, 7-megastigmadien-9-one (4),(-)-10-oxo-isodauc-3-en-15-oic acid (5),isoerivanin (6),eudesmafraglaucolide (7), artanomalide A (8),13-acetoxy-3β-hydroxy-germacra-1(10) E,4E,7(11)-trien-12,6α-olide (9),13-acetoxy-3β-tigloyl-germacra-1(10) E, 4E, 7(11)-trien-12, 6α-olide (10),13-acetoxy-3β-(3-methylbutanoyl)-germacra-1(10)E, 4E, 7(11)-trien-12, 6α-olide (11),3,9-diacetoxy-13-hydroxy-1(10), 4, 7(11)-germacratrien-12,6α-olide (12), and 8α-angeloyloxycostunolide (13). Compounds 1-6 and 13 were obtained from the genus Artemisia for the first time, and 7-9 and 12 were isolated from this plant for the first time. Compound 8 exhibited selective cytotoxicity against human colon cancer (HCT-8) and human gastric cancer (BGC-823) with IC₅₀ values of 2.33 and 4.53 μmol•L ⁻¹, respectively.
Dimeric guaianolides and a fulvenoguaianolide from Artemisia myriantha.[Pubmed: 11975484]
The aerial parts of Artemisia myriantha have afforded one new fulvenoguaianolide and four dimeric guaianolides in addition to seven known guaianolides. The structures of all the compounds were elucidated by 2D NMR. It is speculated that the dimeric guianolides are formed via Diels-Alder type reactions of fulvenoguaianolide derivatives.
Germacranolides from Artemisia myriantha and their conformation.[Pubmed: 11853748]
The CH(2)Cl(2) extract of the aerial parts of Artemisia myriantha afforded three germacranolides derived from 13-acetoxy-3beta-hydroxy-germacra-1(10)E,4E,7(11)-trien-12,6alpha-olide, whose structures were elucidated by 2D-NMR spectroscopic analyses. Some conclusions are drawn about the possible conformations of the ten-membered germacranolide ring system from the exchange peaks seen in the NOESY spectra, and an estimate is made of the energy barrier to ring-flipping from variable-temperature NOESY experiments. The conclusions reached were supported by molecular modeling studies and an NMR spectroscopic investigation of the commercially available germacranolide, parthenolide.
In vitro biological activities of arglabin, a sesquiterpene lactone from the Chinese herb Artemisia myriantha Wall. (Asteraceae).[Pubmed: 8374514]
The immunomodulating properties of arglabin, a sesquiterpene lactone isolated from Artemisia myriantha Wall. (Asteraceae) were investigated using the murine macrophage tumor line J774.1. Arglabin-stimulated macrophages displayed a strong cytotoxic activity and the lowest doses (1.25 micrograms/mL and 0.125 micrograms/mL) induced a significant stimulation of cell mitochondrial metabolism, which correlated with [3H]TdR uptake by J774.1 cells under the same experimental conditions. In addition, the secretion of cytokines involved in host defence mechanisms--IL-1, TNF-alpha, and IL-2--was investigated upon incubation of J774-1 cells with arglabin. Arglabin triggered the production of the three cytokines from J774-1 cells. However, the pattern of cytokine secretion differed to some extent, according to the methodology used for cytokine measurement: either traditional bioassay or specific immunoassay (ELISA). Our data emphasize a possible proliferative effect of arglabin in the traditional bioassays, at least for the highest concentrations used. The results were verified with specific ELISA immunoassays. Using either method, lower concentrations of arglabin (ranging from 12.5 micrograms/mL to 0.125 micrograms/mL) were the most effective in inducing IL-1, TNF-alpha, or IL-2 secretion. In addition, preliminary data on phagocytosis showed that arglabin enhanced the uptake of fluorescent latex beads by J774.1 cells.


