Aralia decaisneana
Aralia decaisneana
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Aralia decaisneana
- Cat.No. Product Name CAS Number COA
-
BCN4327
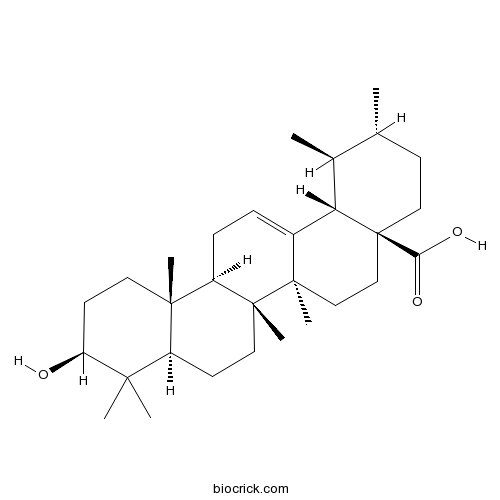
Ursolic acid77-52-1
Instructions
Anti-hepatoma activity and mechanism of ursolic acid and its derivatives isolated from Aralia decaisneana.[Pubmed: 16521214]
To investigate the anti-tumor activity of ursolic acid (UA) and its derivatives isolated from Aralia decaisneana on hepatocellular carcinoma both in vitro and in vivo.
[Morphological and histological identification and essential oil GC-MS assay of Aralia decaisneana].[Pubmed: 12575277]
This paper reported the studies on morphological and histological diagnostic characteristics and powder feature the root of Aralia decaisneana Hance. Chemical analysis indicates essential oil from root cortex contains at least 16 constituents which trans-caryophllene, 9,12-octadecaienoic acid, etc. are the major ingredients.
Araliasaponins I-XI, triterpene saponins from the roots of Aralia decaisneana.[Pubmed: 8729464]
Seven new oleanane-type and four new ursane-type triterpene saponins, named araliasaponins I-XI were isolated from the roots of Aralia decaisneana, together with four known triterpene saponins. On the basis of the chemical and spectroscopic evidence, the structures of these new saponins were elucidated as follows: 3-O-beta-D-xylopyranosyl-(1-->3)-beta-D- glucopyranosyl-(1-->3)-[beta-D-xylopyranosyl-(1-->2)]-alpha-L- arabinopyranosyl oleanolic acid 28-O-beta-D-glucopyranosyl ester, 3-O-beta-D-glucopyranosyl-(1-->3)-alpha-L- arabinopyranosyl oleanolic acid 28-O-beta-D-glucopyranosyl-(1-->6)-beta-D- glucopyranosyl ester, 3-O-beta-D-glucopyranosyl-(1-->3)-[beta-D- xylopyranosyl-(1-->2)]-alpha-L-arabinopyranosyl oleanolic acid 28-O-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranosyl ester, 3-O-beta-D-glucopyranosyl-(1-->3)-[beta-D-xylopyranosyl-(1-->2)]-beta-D- glucopyranosyl oleanolic acid 28-O-beta-D-glucopyranosyl ester, 3-O-beta-D-glucopyranosyl-(1-->3)-[beta-D-xylopyranosyl-(1-->2)]-beta-D- galactopyranosyl oleanolic acid, 3-O-beta-D-glucopyranosyl-(1-->3)-[beta-D- xylopyranosyl-(1-->2)]-beta-D-galactopyranosyl oleanolic acid 28-O-beta-D-glucopyranosyl ester, 3-O-beta-D-glucopyranosyl-(1-->3)-[beta-D-xylopyranosyl-(1-->2)]-beta-D- galactopyranosyl oleanolic acid 28-O-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranosyl ester, 3-O-beta-D-glucopyranosyl-(1-->3)-[beta-D-xylopyranosyl-(1-->2)]- alpha-L-arabinopyranosyl ursolic acid 28-O-beta-D-glucopyranosyl ester, 3-O-beta-D-glucopyranosyl-(1-->3)-[beta-D-xylopyranosyl-(1-->2)]-alpha-L - arabinopyranosyl ursolic acid, 3-O-beta-D-glucopyranosyl-(1-->3)-alpha-L-arabinopyranosyl ursolic acid 28- O-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranosyl ester and 3-O-beta-D-glucopyranosyl-(1-->3)-[beta-D-xylopyranosyl-(1-->2)]- beta-D-glucopyranosyl ursolic acid 28-O-beta-D-glucopyranosyl ester.
[Chemical constituents of Aralia decaisneana Hance].[Pubmed: 7811368]
Six compounds were isolated from the root bark of Aralia decaisneana and elucidated by spectral and chemical analyses as 3-O-[beta-D-galactopyranosyl-(1-->4)-beta-D-galactopyranosyl- (1-->3)-beta-D-glucuronopyranosyl]-oleanolic acid (Ad-V), chikusetsusaponin IVa (Ad-IX), deglucose chikusetsusaponin IVa (Ad-X), palmitic acid (Ad-VI), beta-sitosterol (Ad-VII) and oleanolic acid (Ad-VIII). Ad-V was obtained from nature for the first time, and the rest were all obtained from this plant for the first time.


