ZM 241385Potent, highly selective A2A antagonist CAS# 139180-30-6 |
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Chelerythrine chloride
Catalog No.:BCN8322
CAS No.:3895-92-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 139180-30-6 | SDF | Download SDF |
| PubChem ID | 176407 | Appearance | Powder |
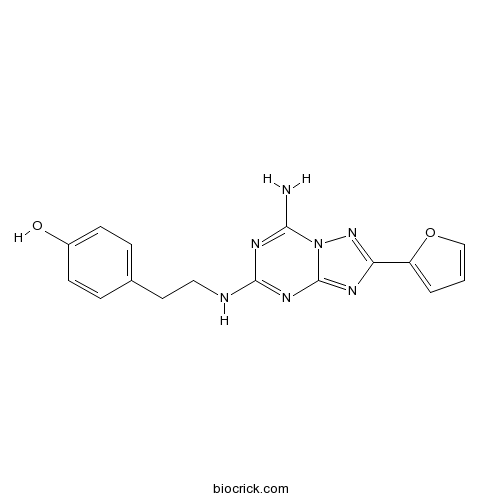
| Formula | C16H15N7O2 | M.Wt | 337.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 30 mg/mL (88.93 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-[2-[[7-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-yl]amino]ethyl]phenol | ||
| SMILES | C1=COC(=C1)C2=NN3C(=NC(=NC3=N2)NCCC4=CC=C(C=C4)O)N | ||
| Standard InChIKey | PWTBZOIUWZOPFT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and highly selective A2A adenosine antagonist, with a pA2 of 9.02 for A2A receptors in guinea pig cardiac vasculature and fold selectivities of 1000, 91 and 500,000 over A1, A2B and A3 sites respectively. Active in vivo. |

ZM 241385 Dilution Calculator

ZM 241385 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9644 mL | 14.8218 mL | 29.6437 mL | 59.2874 mL | 74.1092 mL |
| 5 mM | 0.5929 mL | 2.9644 mL | 5.9287 mL | 11.8575 mL | 14.8218 mL |
| 10 mM | 0.2964 mL | 1.4822 mL | 2.9644 mL | 5.9287 mL | 7.4109 mL |
| 50 mM | 0.0593 mL | 0.2964 mL | 0.5929 mL | 1.1857 mL | 1.4822 mL |
| 100 mM | 0.0296 mL | 0.1482 mL | 0.2964 mL | 0.5929 mL | 0.7411 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ZM 241385 is a novel non-xanthine adenosine receptor antagonist with selectivity for the A2a receptor subtype. Target: adenosine receptor in vitro: ZM 241385 has high affinity for A2a receptors. In rat phaeochromocytoma cell membranes, ZM 241385 displaces binding of tritiated 5'-N-ethylcarboxamidoadenosine (NECA) with a pIC50 of 9.52. [1] in vivo: ZM 241385 has low potency at A2b receptors and antagonized the relaxant effects of adenosine in the guinea-pig aorta. ZM 241385 has a low affinity at A1 receptors. In rat cerebral cortex membranes it displaces tritiated R-phenylisopropyladenosine (R-PIA) with a pIC50 of 5.69. [2]
References:
[1]. Poucher SM, et al. The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br J Pharmacol. 1995 Jul;115(6):1096-102.
- CEP-37440
Catalog No.:BCC5145
CAS No.:1391712-60-9
- H-Tle-OH.HCl
Catalog No.:BCC2660
CAS No.:139163-43-2
- Uralenol
Catalog No.:BCN7994
CAS No.:139163-15-8
- Tripterifordin
Catalog No.:BCN6206
CAS No.:139122-81-9
- Isomurralonginol acetate
Catalog No.:BCN4708
CAS No.:139115-59-6
- BM-1074
Catalog No.:BCC2235
CAS No.:1391108-10-3
- Zanamivir
Catalog No.:BCC4946
CAS No.:139110-80-8
- Vigabatrin Hydrochloride
Catalog No.:BCC5198
CAS No.:1391054-02-6
- PF 4800567 hydrochloride
Catalog No.:BCC7904
CAS No.:1391052-28-0
- Tristin
Catalog No.:BCN4709
CAS No.:139101-67-0
- (-)-Heraclenol
Catalog No.:BCN7682
CAS No.:139079-42-8
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- Picfeltarraenin X
Catalog No.:BCN2859
CAS No.:1391826-61-1
- Verdinexor (KPT-335)
Catalog No.:BCC5573
CAS No.:1392136-43-4
- 24-Hydroxy-25-ethoxy-3,4-secocycloart-4(28)-en-3-oic acid methyl ester
Catalog No.:BCN7050
CAS No.:1392210-81-9
- Dodonaflavonol
Catalog No.:BCN6862
CAS No.:1392213-93-2
- 3,4-Dihydroxy-2-O-methylanigorufone
Catalog No.:BCN7182
CAS No.:1392307-42-4
- Musellarin B
Catalog No.:BCN7192
CAS No.:1392476-32-2
- Musellarin C
Catalog No.:BCN7004
CAS No.:1392476-33-3
- G-36
Catalog No.:BCC6283
CAS No.:1392487-51-2
- Fmoc-Lys-OH.HCl
Catalog No.:BCC3512
CAS No.:139262-23-0
- H2L5186303
Catalog No.:BCC6315
CAS No.:139262-76-3
- Zolmitriptan
Catalog No.:BCC5062
CAS No.:139264-17-8
- MDL 100907
Catalog No.:BCC7877
CAS No.:139290-65-6
The A2a/A2b receptor antagonist ZM-241385 blocks the cardioprotective effect of adenosine agonist pretreatment in in vivo rat myocardium.[Pubmed:16980350]
Am J Physiol Heart Circ Physiol. 2007 Jan;292(1):H426-31.
There is increasing evidence for interactions among adenosine receptor subtypes in the brain and heart. The purpose of this study was to determine whether the adenosine A(2a) receptor modulates the infarct size-reducing effect of preischemic administration of adenosine receptor agonists in intact rat myocardium. Adult male rats were submitted to in vivo regional myocardial ischemia (25 min) and 2 h reperfusion. Vehicle-treated rats were compared with rats pretreated with the A(1) agonist 2-chloro-N(6)-cyclopentyladenosine (CCPA, 10 mug/kg), the nonselective agonist 5'-N-ethylcarboxamidoadenosine (NECA, 10 mug/kg), or the A(2a) agonist 2-[4-(2-carboxyethyl)phenethylamino]-5'-N-methylcarboxamidoadenosine (CGS-21680, 20 mug/kg). Additional CCPA- and NECA-treated rats were pretreated with the A(1) antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, 100 mug/kg), the A(2a)/A(2b) antagonist 4-(-2-[7-amino-2-{2-furyl}{1,2,4}triazolo{2,3-a} {1,3,5}triazin-5-yl-amino]ethyl)phenol (ZM-241385, 1.5 mg/kg) or the A(3) antagonist 3-propyl-6-ethyl-5[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridine carboxylate (MRS-1523, 2 mg/kg). CCPA and NECA reduced myocardial infarct size by 50% and 35%, respectively, versus vehicle, but CGS-21680 had no effect. DPCPX blunted the bradycardia associated with CCPA and NECA, whereas ZM-241385 attenuated their hypotensive effects. Both DPCPX and ZM-241385 blocked the protective effects of CCPA and NECA. The A(3) antagonist did not alter the hemodynamic effects of CCPA or NECA, nor did it alter adenosine agonist cardioprotection. None of the antagonists alone altered myocardial infarct size. These findings suggest that although preischemic administration of an A(2a) receptor agonist does not induce cardioprotection, antagonism of the A(2a) and/or the A(2b) receptor blocks the cardioprotection associated with adenosine agonist pretreatment.
Characterization of [125I]ZM 241385 binding to adenosine A2A receptors in the pineal of sheep brain.[Pubmed:16764836]
Brain Res. 2006 Jun 22;1096(1):30-9.
Adenosine is a ubiquitous neuromodulator and homeostatic regulator that exerts its physiologic actions through activation of A(1), A(2A), A(2B) and A(3) adenosine receptor subtypes. In the central nervous system, adenosine's action in neurons is manifested in its modulation of tonic inhibitory control. Adenosine released in the brain during hypoxia has critical depressant effects on breathing in fetal and newborn mammals, an action suggested to be mediated by A(2A) receptors in the posteromedial thalamus. In an effort to more accurately define the spatial distribution of adenosine A(2A) receptors in fetal sheep diencephalon, we have used a receptor autoradiographic technique utilizing an iodinated radioligand [(125)I]ZM 241385, which has greater sensitivity and resolution than the tritiated compound. The distribution of ligand binding sites in the fetal sheep diencephalon indicated that the highest levels of binding were in select thalamic nuclei, including those implicated in hypoxic depression of fetal breathing, and the pineal. Given the high density of labeled A(2A) receptors in the pineal, these sites were characterized more fully in homogenate radioligand binding assays. These data indicate that [(125)I]ZM 241385 binding sites display a pharmacological signature consistent with that of adenosine A(2A) receptors and are expressed at similar levels in fetal, lamb and adult ovine brain. The adenosine A(2A) receptor pharmacologic signature of the [(125)I]ZM 241385 binding site in pineal cell membranes generalized to the site characterized in membranes derived from other portions of the lamb thalamus, including the sector involved in hypoxic inhibition of fetal breathing. These results have important implications for the functional roles of adenosine A(2A) receptors in the thalamus and pineal of sheep brain.
Characterization of [3H]ZM 241385 binding in wild-type and adenosine A2A receptor knockout mice.[Pubmed:15507221]
Eur J Pharmacol. 2004 Nov 3;504(1-2):55-9.
The binding of the adenosine A(2A) receptor antagonist [3H] 4-(2-[7-amino-2-(2-furyl)[1,2,4]-triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)ph enol ([3H]ZM 241385) to mouse brain and spinal cord was investigated. In brain homogenates, single-site binding was observed with a Bmax of 299+/-28 fmol mg(-1) protein and a Kd of 0.75+/-0.08 nM. In autoradiographic studies, there was a high density of specific binding of [3H]ZM 241385 in the striatum, with a very low density in the cortex and no binding elsewhere in the brain or in the spinal cord. All specific binding of [3H]ZM 241385 was lost in genetically modified mice lacking the adenosine A(2A) receptor, confirming the selectivity of this radioligand.
Region-specific neuroprotective effect of ZM 241385 towards glutamate uptake inhibition in cultured neurons.[Pubmed:19619523]
Eur J Pharmacol. 2009 Sep 1;617(1-3):28-32.
Active uptake by neurons and glial cells is the main mechanism for maintaining extracellular glutamate at low, non-toxic concentrations. Adenosine A(2A) receptors regulate extracellular glutamate levels by acting on both the release and the uptake of glutamate. The aim of this study was to evaluate whether the inhibition of the effects of glutamate uptake blockers by adenosine A(2A) receptor antagonists resulted in neuroprotection. In cortical and striatal neuronal cultures, the application of l-trans-pyrrolidine-2,4-dicarboxylic acid (PDC, a transportable competitive inhibitor of glutamate uptake), induced a dose-dependent increase in lactate dehydrogenase (LDH) levels, an index of cytotoxicity. Such an effect of PDC was significantly reduced by pre-treatment with the adenosine A(2A) receptor antagonist ZM 241385 (50 nM) in striatal, but not cortical, cultures. The protective effects of ZM 241385 were specifically due to a counteraction of PDC effects, since ZM 241385 was totally ineffective in preventing the cytotoxicity induced by direct application of glutamate to cultures. These results indicate that adenosine A(2A) receptor antagonists prevent the toxic effects induced by a transportable competitive inhibitor of glutamate uptake, that such an effect specifically occurs in the striatum and that it does not depend on a direct blockade of glutamate-induced toxicity.
In vivo characterisation of ZM 241385, a selective adenosine A2A receptor antagonist.[Pubmed:8773453]
Eur J Pharmacol. 1996 Apr 22;301(1-3):107-13.
The in vivo characterisation of ZM 241385 (4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-+ ++ylamino] ethyl)phenol), a novel, non-xanthine, selective adenosine A2A antagonist is described. In anaesthetised dogs ZM 241385 (i.v.) was 140-fold more potent in attenuating vasodilator responses to exogenous adenosine in the constant flow perfused hind limb than the bradycardic effects. In pithed rats in which blood pressure was supported by angiotensin II infusion, ZM 241385 (10 mg kg-1, i.v.) did not inhibit the hypotensive or bradycardic effects of the A3/A1 receptor agonist N(6)-2-(4-amino-3-iodophenyl)ethyladenosine (APNEA). In conscious spontaneously hypertensive rats, ZM 241385 (3-10 mg kg-1, p.o.) selectively attenuated the mean arterial blood pressure response produced by exogenous adenosine. No inhibition of the bradycardic effects of adenosine was observed following these doses of ZM 241385. The results indicate that ZM 241385 can be used to evaluate the role of adenosine A2A receptors in the action of adenosine in vivo.
Pharmacodynamics of ZM 241385, a potent A2a adenosine receptor antagonist, after enteric administration in rat, cat and dog.[Pubmed:8832494]
J Pharm Pharmacol. 1996 Jun;48(6):601-6.
4-(2-[7-Amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5] triazin-5-ylamino]ethyl)phenol (ZM 241385) is currently the most selective for the A2a adenosine receptor antagonist. This paper describes the in-vivo activity of ZM 241385 after administration by both oral and intraduodenal routes. In conscious spontaneously hypertensive rats, ZM 241385 (1-10 mg kg-1) selectively attenuated the mean arterial blood pressure response produced by exogenous adenosine (1 mg kg-1 min-1, i.v.) by up to 45% after oral administration. Activity of ZM 241385 was maintained for at least 6 h after 3 and 10 mg kg-1 (p.o.). In conscious normotensive cats, ZM 241385 attenuated the blood pressure responses to adenosine (0.6-1.0 mg kg-1, i.v.) by 94% after 10 mg kg-1 (p.o.) and by up to 74% after 0.3 mg kg-1 (i.v.). Duration of action of ZM 241385 up to 12 h (36% inhibition) was observed after 3 mg kg-1 (p.o.). In anaesthetized dogs and cats, ZM 241385, after intraduodenal administration (1-10 mg kg-1), produced a rapid (dose ratio 100-fold 15 min after administration of 10 mg kg-1 in the cat) and prolonged (dose ratio of 14 at 6 h after administration of 10 mg kg-1) attenuation of the vasodilatation responses to adenosine receptor stimulation. When administered by this route ZM 241385 was six times more potent than theophylline in the cat and at least twice as potent as theophylline in the dog. In conclusion, ZM 241385 is a potent, selective A2a adenosine receptor antagonist which is orally active, with a good duration of action by the enteric route in cat, rat and dog. It could therefore be used to evaluate the role of adenosine A2a receptors in the action of adenosine in-vivo.
The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist.[Pubmed:7582508]
Br J Pharmacol. 1995 Jul;115(6):1096-102.
1. This paper describes the in vitro pharmacology of ZM 241385 (4-(2-[7-amino-2-(2-furyl) [1,2,4]-triazolo[2,3-a][1,3,5]triazin- 5-yl amino]ethyl) phenol), a novel non-xanthine adenosine receptor antagonist with selectivity for the A2a receptor subtype. 2. ZM 241385 had high affinity for A2a receptors. In rat phaeochromocytoma cell membranes, ZM 241385 displaced binding of tritiated 5'-N-ethylcarboxamidoadenosine (NECA) with a pIC50 of 9.52, (95% confidence limits, c.l., 9.02-10.02). In guinea-pig isolated Langendorff hearts, ZM 241385 antagonized vasodilatation of the coronary bed produced by 2-chloroadenosine (2-CADO) and 2-[p-(2-carboxyethyl) phenethylamino]-5'-N-ethylcarboxamidoadenosine (CGS21680) with pA2 values of 8.57 (c.l., 8.45-8.68) and 9.02 (c.l., 8.79-9.24) respectively. 3. ZM 241385 had low potency at A2b receptors and antagonized the relaxant effects of adenosine in the guinea-pig aorta with a pA2 of 7.06, (c.l., 6.92-7.19). 4. ZM 241385 had a low affinity at A1 receptors. In rat cerebral cortex membranes it displaced tritiated R-phenylisopropyladenosine (R-PIA) with a pIC50 of 5.69 (c.l., 5.57-5.81). ZM 241385 antagonized the bradycardic action of 2-CADO in guinea-pig atria with a pA2 of 5.95 (c.l., 5.72-6.18). 5. ZM 241385 had low affinity for A3 receptors. At cloned rat A3 receptors expressed in chinese hamster ovary cells, it displaced iodinated aminobenzyl-5'-N-methylcarboxamido adenosine (AB-MECA) with a pIC50 of 3.82 (c.l., 3.67-4.06). 6. ZM 241385 had no significant additional pharmacological effects on the isolated tissues used in these studies at concentrations three orders of magnitude greater than those which block A2a receptors. At 10 microM it displayed only minor inhibition of the bradycardic effects in guinea-pig atria to some concentrations of carbachol. At 10 microM, ZM 241385 had a small inhibitory effect on relaxant effects of isoprenaline in guinea-pig aortae but no effect on sodium nitrite-induced relaxation. ZM 241385(100 microM) was without effect on phenylephrine-induced tone in guinea-pig aortae.7. ZM 241385 (10 microM) had no inhibitory effect on rat hepatocyte phosphodiesterase types I, II, III and IV but caused a small inhibition of the calcium calmodulin-activated type I enzyme.8. ZM 241385 is the most selective adenosine A2a receptor antagonist yet described and is therefore a useful tool for characterization of responses mediated by A2 adenosine receptors.


