Vincetoxicoside BCAS# 22007-72-3 |
Quality Control & MSDS
Number of papers citing our products

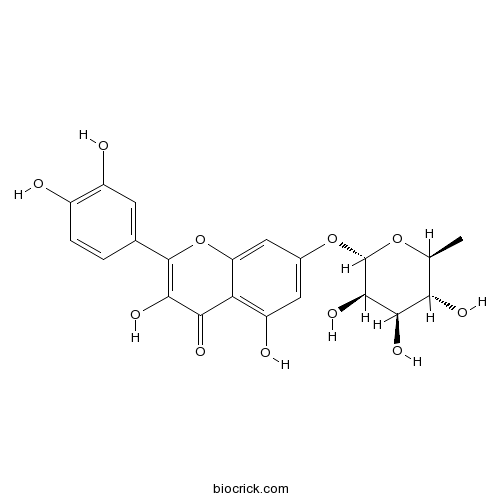
Chemical structure

3D structure
| Cas No. | 22007-72-3 | SDF | Download SDF |
| PubChem ID | 5748601 | Appearance | Yellow powder |
| Formula | C21H20O11 | M.Wt | 448.38 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 7-Rhamnosylquercetin; 3,3',4',5,7-Pentahydroxyflavone 7-rhamnoside; Vincetoxicoside B | ||
| Solubility | Soluble in methanol; slightly soluble in water | ||
| Chemical Name | 2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-7-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)OC2=CC(=C3C(=C2)OC(=C(C3=O)O)C4=CC(=C(C=C4)O)O)O)O)O)O | ||
| Standard InChIKey | QPHXPNUXTNHJOF-XNFUJFQVSA-N | ||
| Standard InChI | InChI=1S/C21H20O11/c1-7-15(25)17(27)19(29)21(30-7)31-9-5-12(24)14-13(6-9)32-20(18(28)16(14)26)8-2-3-10(22)11(23)4-8/h2-7,15,17,19,21-25,27-29H,1H3/t7-,15-,17+,19+,21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vincetoxicoside B , quercetin, kaempferol , and (-)-epicatechin show synergistic antifungal activities with the FICI values <0.5. |
| Targets | Antifection |
| In vitro | Chemical constituents from the rhizome of Polygonum paleaceum and their antifungal activity.[Pubmed: 27309618 ]J Asian Nat Prod Res. 2017 Jan;19(1):47-52.
|
| Structure Identification | J Pharm Biomed Anal. 2016 Jan 25;118:228-34.Simultaneous determination and pharmacokinetic study of eight components in rat plasma by UHPLC-MS/MS after oral administration of Hypericum japonicum Thunb extract.[Pubmed: 26580819 ]
|

Vincetoxicoside B Dilution Calculator

Vincetoxicoside B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2303 mL | 11.1513 mL | 22.3025 mL | 44.605 mL | 55.7563 mL |
| 5 mM | 0.4461 mL | 2.2303 mL | 4.4605 mL | 8.921 mL | 11.1513 mL |
| 10 mM | 0.223 mL | 1.1151 mL | 2.2303 mL | 4.4605 mL | 5.5756 mL |
| 50 mM | 0.0446 mL | 0.223 mL | 0.4461 mL | 0.8921 mL | 1.1151 mL |
| 100 mM | 0.0223 mL | 0.1115 mL | 0.223 mL | 0.4461 mL | 0.5576 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- Ixabepilone
Catalog No.:BCC1666
CAS No.:219989-84-1
- Fmoc-β-Homo-Tyr(tBu)-OH
Catalog No.:BCC2621
CAS No.:219967-69-8
- Isoescin IB
Catalog No.:BCN2969
CAS No.:219944-46-4
- Isoescin IA
Catalog No.:BCN2968
CAS No.:219944-39-5
- 3-Methyl-1-(2-piperidinophenyl)butylamine N-acetylglutamate salt
Catalog No.:BCC8635
CAS No.:219921-94-5
- 5,8,9,14-Tetraacetoxy-3-benzoyloxy-10,15-dihydroxypepluane
Catalog No.:BCN7657
CAS No.:219916-77-5
- MPEP Hydrochloride
Catalog No.:BCC1777
CAS No.:219911-35-0
- 2,2',3'-Trihydroxy-4,6-dimethoxybenzophenone
Catalog No.:BCN1488
CAS No.:219861-73-1
- Escitalopram Oxalate
Catalog No.:BCC5040
CAS No.:219861-08-2
- Merresectine B
Catalog No.:BCN1918
CAS No.:219829-75-1
- Consiculine
Catalog No.:BCN1903
CAS No.:219829-73-9
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Eichlerialactone
Catalog No.:BCN4941
CAS No.:2202-01-9
- O-Methyldauricine
Catalog No.:BCC8225
CAS No.:2202-17-7
- Triptohairic acid
Catalog No.:BCN8060
CAS No.:220209-71-2
- Cinnamamide
Catalog No.:BCN4942
CAS No.:22031-64-7
- 5-[2-[Tert-butyl(dimethyl)silyl]oxyethyl]-2,2-dimethyl-3a,6a-dihydrofuro[2,3-d][1,3]dioxol-6-one
Catalog No.:BCC8592
CAS No.:220328-03-0
- 3,11,12-Trihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1487
CAS No.:220328-04-1
- TRO 19622
Catalog No.:BCC5288
CAS No.:22033-87-0
- DH 97
Catalog No.:BCC6973
CAS No.:220339-00-4
- 5-Epicanadensene
Catalog No.:BCN7349
CAS No.:220384-17-8
- ShK-Dap22
Catalog No.:BCC5990
CAS No.:220384-25-8
- Polycephalin C
Catalog No.:BCN1852
CAS No.:220422-37-7
Chemical constituents from the rhizome of Polygonum paleaceum and their antifungal activity.[Pubmed:27309618]
J Asian Nat Prod Res. 2017 Jan;19(1):47-52.
A new compounds neopaleaceolactoside (1), along with nine known compounds phyllocoumarin (2), quercetin (3), quercitrin (4), quercetin-3-methyl ether (5), Vincetoxicoside B (6), isoquercitrin (7), kaempferol (8), (-)-epicatechin (9), and chlorogenic acid (10), was isolated from Polygonum paleaceum Wall. Their chemical structures were established based on one-dimensional and two-dimensional nuclear magnetic resonance techniques, mass spectrometry and by comparison with spectroscopic data reported. Some selected compounds were screened for their antifungal activity. Quercetin (3), Vincetoxicoside B (6), kaempferol (8), and (-)-epicatechin (9) showed synergistic antifungal activities with the FICI values <0.5. A preliminary structure-activity relationship could be observed that free 3-OH in the structure of flavonoids was important for synergistic antifungal activity.
Simultaneous determination and pharmacokinetic study of eight components in rat plasma by UHPLC-MS/MS after oral administration of Hypericum japonicum Thunb extract.[Pubmed:26580819]
J Pharm Biomed Anal. 2016 Jan 25;118:228-234.
A rapid and sensitive assay based on ultra high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) was established and validated for the simultaneous determination of gallic acid, protocatechuic acid, vanillic acid, caffeic acid, epicatechin, isoquercitrin, Vincetoxicoside B and quercetin in rat plasma using catechin and daidzein as the internal standards (IS). Plasma samples added internal standards were acidified with formic acid then pretreated by direct protein precipitation with acetonitrile. The separation of eight constituents was achieved on a C18 column with gradient elution using methanol and 0.2% acetic acid aqueous solution as the mobile phase and detected by multiple reaction monitoring using electrospray ionization source in the positive-negative ionization mode. The method was validated for sufficient specificity, precision, accuracy, and sensitivity over the concentration range of 10-6000 ng mL(-1) for gallic acid, 1.5-3000 ng mL(-1) for protocatechuic acid, 10-15000 ng mL(-1) for vanillic acid, 2-3600 ng mL(-1) for caffeic acid, 1.5-3600 ng mL(-1) for epicatechin, 4-6000 ng mL(-1) for isoquercitrin, 2-9000 ng mL(-1) for Vincetoxicoside B, and 20-18000 ng mL(-1) for quercetin. The overall intrarun precision and the interrun precision were showed in the range of 1.0-14.2% and 2.8-12.9%, respectively, and the accuracy was no more than 12.8%. This analytical method was successfully applied to investigate the pharmacokinetics of eight ingredients in rats after oral administration of Hypericum japonicum Thunb extract.


