UmckalinCAS# 43053-62-9 |
Quality Control & MSDS
Number of papers citing our products

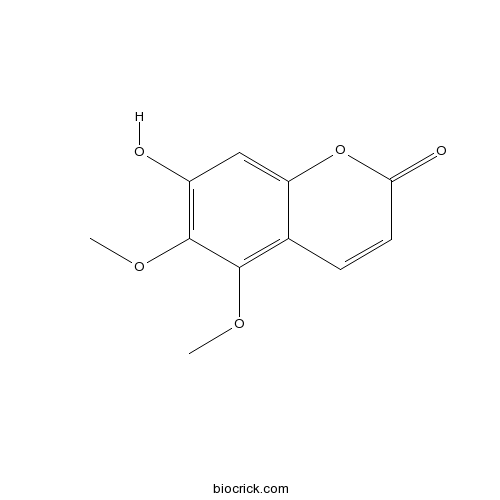
Chemical structure

3D structure
| Cas No. | 43053-62-9 | SDF | Download SDF |
| PubChem ID | 5316862 | Appearance | White powder |
| Formula | C11H10O5 | M.Wt | 222 |
| Type of Compound | Phenylpropanes | Storage | Desiccate at -20°C |
| Synonyms | 7-Hydroxy 5,6-dimethoxycoumarin | ||
| Solubility | Soluble in methanol; slightly soluble in water | ||
| Chemical Name | 7-hydroxy-5,6-dimethoxychromen-2-one | ||
| SMILES | COC1=C2C=CC(=O)OC2=CC(=C1OC)O | ||
| Standard InChIKey | DVBPETFXQYSHLJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H10O5/c1-14-10-6-3-4-9(13)16-8(6)5-7(12)11(10)15-2/h3-5,12H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Umckalin Dilution Calculator

Umckalin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5045 mL | 22.5225 mL | 45.045 mL | 90.0901 mL | 112.6126 mL |
| 5 mM | 0.9009 mL | 4.5045 mL | 9.009 mL | 18.018 mL | 22.5225 mL |
| 10 mM | 0.4505 mL | 2.2523 mL | 4.5045 mL | 9.009 mL | 11.2613 mL |
| 50 mM | 0.0901 mL | 0.4505 mL | 0.9009 mL | 1.8018 mL | 2.2523 mL |
| 100 mM | 0.045 mL | 0.2252 mL | 0.4505 mL | 0.9009 mL | 1.1261 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Deoxyshikonin
Catalog No.:BCN3006
CAS No.:43043-74-9
- Vinpocetine
Catalog No.:BCN2609
CAS No.:42971-09-5
- Cathepsin G Inhibitor I
Catalog No.:BCC3598
CAS No.:429676-93-7
- Dabigatran ethyl ester
Catalog No.:BCC1512
CAS No.:429658-95-7
- Nabumetone
Catalog No.:BCC4434
CAS No.:42924-53-8
- Z-Abu-OH
Catalog No.:BCC3201
CAS No.:42918-86-5
- Cladribine
Catalog No.:BCC1173
CAS No.:4291-63-8
- Tilianin
Catalog No.:BCN3669
CAS No.:4291-60-5
- Santamarine
Catalog No.:BCN5485
CAS No.:4290-13-5
- 14-Deoxy-11,12-didehydroandrographolide
Catalog No.:BCN1441
CAS No.:42895-58-9
- For-Met-OH
Catalog No.:BCC2992
CAS No.:4289-98-9
- 2-(3-Benzoylphenyl)propionitrile
Catalog No.:BCC8479
CAS No.:42872-30-0
- DPO-1
Catalog No.:BCC7398
CAS No.:43077-30-1
- Necrostatin-1
Catalog No.:BCC2247
CAS No.:4311-88-0
- 7'-O-Ethylmarmin
Catalog No.:BCC8274
CAS No.:
- SJ 172550
Catalog No.:BCC2416
CAS No.:431979-47-4
- Allylestrenol
Catalog No.:BCC8814
CAS No.:432-60-0
- Zopiclone
Catalog No.:BCC9195
CAS No.:43200-80-2
- 6-(5-Chloropyridin-2-yl)-7-hydroxy-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-one
Catalog No.:BCC8753
CAS No.:43200-81-3
- Skp2 Inhibitor C1
Catalog No.:BCC6298
CAS No.:432001-69-9
- Fenbendazole
Catalog No.:BCC1236
CAS No.:43210-67-9
- Formoterol Hemifumarate
Catalog No.:BCC4349
CAS No.:43229-80-7
- 6-Hydroxykaempferol
Catalog No.:BCN3334
CAS No.:4324-55-4
- Boc-Tyr-OMe
Catalog No.:BCC3459
CAS No.:4326-36-7
An Overview of Phytotherapeutic Approaches for the Treatment of Tuberculosis.[Pubmed:27145855]
Mini Rev Med Chem. 2017;17(2):167-183.
Tuberculosis (TB) is highly infectious disease causing morbidity and death. Its causative organism is a contagious bacterium, Mycobacterium tuberculosis. The incidence of tuberculosis is increasing worldwide due to the emergence of drug resistance bacteria. The resistance is being developed in Mycobacterium tuberculosis against both the first line as well as the second line drugs used for the treatment. The tuberculosis control programme is being complicated and failed to get the desired impact due to the development of multi-drug resistant (MDR) and extensively-drug resistant (XDR) strains of Mycobacterium tuberculosis. So, there is a critical requirement to discover and produce newer anti-TB drugs with unique drug targets. Medicinal plants have been used for curing the diseases from ancient time. Medicinal plants are the novel sources for the production of alternate medicines for the treatment of TB caused by MDR and XDR strains. Plants produce a number of different kinds of secondary metabolites such as Alkaloids, Coumarins, Flavonoids, Polyphenols, Terpenoids, Quinines, etc. which have antimicrobial activity; thus may be useful in control of tuberculosis. These compounds do not contribute directly in growth and development but used by the plants for their defense. On the basis of various sources in the literature, about 72 phytochemicals constituents responsible for anti tubercular activity isolated from different plants have been explained along with their structure. Most effective isolated compounds from plants are plumbagin, maritinone, 3, 3'-biplumbagin, aloe emodin, epigallocatechin and Umckalin. These phytochemicals are helpful for the treatment of MDR, XDR type of tuberculosis. This review describes an overview of the current synthetic medicines used for treatment of TB and the work carried out on anti tubercular plants along with their phytochemicals.
Reproduction of the Medicinal Plant Pelargonium sidoides via Somatic Embryogenesis.[Pubmed:26287694]
Planta Med. 2015 Aug;81(12-13):1169-74.
The medicinal plant Pelargonium sidoides DC. (Geraniaceae) was traditionally used for the treatment of the common cold and cough in South Africa. Today an aequous-ethanolic root extract from this plant is approved for the treatment of acute bronchitis and is globally marketed also as an immunostimulant. The increasing demand of the plant material for the industrial production indicates the need of new effective methods for the propagation of P. sidoides. Here we report somatic embryogenesis and in vitro plantlet regeneration from somatic cells of inflorescence shoots and petioles of P. sidoides. A one-week cultivation of explants in media containing different concentrations of thidiazuron (1, 2.2, 3, and 4 mg/L) followed by a cultivation period without phytohormones resulted in the induction of somatic embryos within 2-4 weeks. After 2-4 months, the embryos generated roots and could be transferred into a greenhouse, where flower formation took place and the development of seeds occurred with high germination rates. The root Umckalin concentration, determined by high-performance thin-layer chromatography, was comparable to that of seed-cultivated plants (100 +/- 6 vs. 113 +/- 10 microg Umckalin/g dried roots). For the first time, direct somatic embryogenesis has been established as an appropriate cultivation method for P. sidoides plants used as raw material in the pharmaceutical industry. Moreover, genetically identical plants (chemical races) can be easily generated by this procedure.
[Chemical constituents of Datura stramonium seeds].[Pubmed:22568232]
Zhongguo Zhong Yao Za Zhi. 2012 Feb;37(3):319-22.
OBJECTIVE: To study chemical constituents in the seeds of Datura Stramonium (Solanaceae family). METHOD: Compounds were isolated and purified by silica gel, MCI and Sephadex LH-20 column chromatography, and their structures were determined based on physicochemical constants and spectroscopic analysis including NMR and MS. RESULT: Twelve compounds were isolated and identified from Datura stramonium, they were N-trans-feruloyl tryptamine (1), hyoscyamilactol (2), scopoletin (3), Umckalin (4), daturaolone (5), daturadiol (6), N-trans-ferulicacyl- tyramine (7), cleomiscosin A (8), fraxetin (9), scopolamine (10), 1-Acetyl-7-hydrox-beta-carbol-ine (11), 7-hydroxy-beta-carbolinel-propionic acid (12). CONCLUSION: Compound 2, 7, 9 and 12 were obtained from Datura genus for the first time, whereas compound 1, 4, 8 and 11 were obtained from the Solanaceae family for the first time.
Determination of umckalin in commercial tincture and phytopreparations containing Pelargonium sidoides by HPLC: comparison of sample preparation procedures.[Pubmed:20441909]
Talanta. 2010 Jun 15;81(4-5):1368-72.
Roots of Pelargonium sidoides D.C. are used for the production of phytomedicines. Current quality control of phytopreparations containing P. sidoides extracts has been made in terms of total phenolics content. In this work we describe the development and validation of an HPLC method for the analysis of P. sidoides tincture and commercial syrup phytopreparations using Umckalin (7-hydroxy-5,6-dimethoxycoumarin) as chemical marker. Two sample preparation procedures, liquid-liquid extraction (LLE) and solid-phase extraction (SPE) were also developed and compared. The samples were analyzed by RP-HPLC and the two methods were then validated and compared. The repeatability of the two procedures showed coefficients of variation (CV) of 1.2% for SPE procedure, and 1.3% for LLE. Recovery for both methods was higher than 95.2%. The linearity showed correlation coefficients better than 0.999 for both methods. The detection and quantification limit were 0.0098 and 0.0298microgmL(-1), respectively. The validated procedure was then used for the analysis of tincture and five batches of two commercial phytopreparations containing P. sidoides tincture.
Nitric oxide synthase and cytokines gene expression analyses in Leishmania-infected RAW 264.7 cells treated with an extract of Pelargonium sidoides (Eps 7630).[Pubmed:16920512]
Phytomedicine. 2006 Sep;13(8):570-5.
A modern aqueous-ethanolic formulation of the roots of Pelargonium sidoides (Eps 7630), elaborated from the traditional herbal medicine used in areas of southern Africa, is effectively employed for the treatment of ENT and respiratory tract infections in modern phytotherapy. Previous studies have demonstrated antibacterial and immunomodulatory activities. To gain insight into the mode of action at the molecular level, gene expression analyses for the inducible nitric oxide synthase and the cytokines interleukin (IL)-1, IL-12, IL-18, tumour necrosis factor (TNF)-alpha, interferon (IFN)-alpha, and IFN-gamma, were performed using reverse transcription-polymerase chain reaction (RT-PCR). The experiments were carried out in parallel in non-infected and in Leishmania major-infected RAW 264.7 cells and the expression profiles were compared with those mediated by IFN-gamma+LPS. Eps 7630 induced low mRNA levels in non-infected cells, and it considerably up-regulated the transcript expressions in parasitised cells. Interestingly, and in contrast to activation by IFN-gamma+LPS, Eps 7630 also stimulated infected cells to produce IFN-gamma mRNA. A similar expression profile was observed for the methanol-insoluble fraction (MIF) of Eps 7630 and gallic acid, a trace constituent of the extract, while the methanol-soluble fraction and Umckalin, an exclusive and representative member of the occurring coumarins, proved to be devoid of any remarkable gene-inducing capabilities. The present results provide not only convincing support for the improvement of immune functions as previously demonstrated in functional bioassays, but also evidence for activation at the transcriptional level and suggest that the underlying inducing principle is located in the MIF.
Antibacterial activity of extracts and constituents of Pelargonium sidoides and Pelargonium reniforme.[Pubmed:9434601]
Planta Med. 1997 Dec;63(6):508-10.
The antibacterial activity of extracts and isolated constituents (scopoletin, Umckalin, 5,6,7-trimethoxycoumarin, 6,8-dihydroxy-5,7-dimethoxycoumarin, (+)-catechin, gallic acid and its methyl ester) of Pelargonium sidoides and Pelargonium reniforme (Geraniaceae), plant species used in folk medicine by the Southern African native population, was evaluated against 8 microorganisms, including 3 Gram-positive (Staphylococcus aureus, Streptococcus pneumoniae, and beta-hemolytic Streptococcus 1451) and 5 Gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Haemophilus influenzae). Minimum inhibitory concentrations (MICs) varied with the preparation of the extracts and microorganisms tested, from about 0.6 mg/ml for aqueous phases to over 10 mg/ml for crude Pelargonium extracts. With the exception of the ineffective (+)-catechin, all the potentially active compounds exhibited antibacterial activities with MICs of 200-1000 micrograms/ml. The results provide for a rational basis of the traditional use of the titled Pelargonium species.
24-norhopene derivatives from Diatenopteryx sorbifolia.[Pubmed:9322363]
J Nat Prod. 1997 Sep;60(9):909-11.
Two new hopene derivatives, 3 beta,6 beta-dihydroxy-21 alpha H-24-norhopa-4(23),22(29)-diene (1) and 3 beta,5 beta-dihydroxy-6 beta-[(4-hydroxybenzoyl)oxy]-21 alpha H-24-norhopa-4(23),22(29)-diene (2), together with cleomiscosin B (3) and 5,6-dimethoxy-7-hydroxycoumarin (Umckalin), were isolated from the timber of Diatenopteryx sorbifolia. This is the first isolation of the norhopene skeleton from nature. The structures of the isolates were established by spectroscopic analysis.


