TirofibanCAS# 144494-65-5 |
- Tirasemtiv
Catalog No.:BCC5183
CAS No.:1005491-05-3
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- TPT-260 Dihydrochloride
Catalog No.:BCC5172
CAS No.:2076-91-7
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Amifampridine
Catalog No.:BCC5185
CAS No.:54-96-6
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
Quality Control & MSDS
Number of papers citing our products

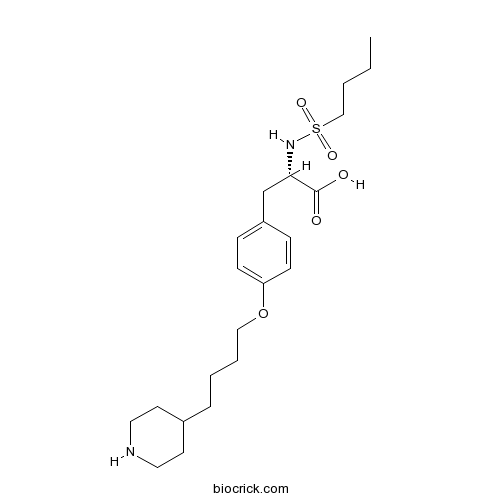
Chemical structure

3D structure
| Cas No. | 144494-65-5 | SDF | Download SDF |
| PubChem ID | 60947 | Appearance | Powder |
| Formula | C22H36N2O5S | M.Wt | 440.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | L700462; MK383 | ||
| Solubility | Limited solubility | ||
| Chemical Name | (2S)-2-(butylsulfonylamino)-3-[4-(4-piperidin-4-ylbutoxy)phenyl]propanoic acid | ||
| SMILES | CCCCS(=O)(=O)NC(CC1=CC=C(C=C1)OCCCCC2CCNCC2)C(=O)O | ||
| Standard InChIKey | COKMIXFXJJXBQG-NRFANRHFSA-N | ||
| Standard InChI | InChI=1S/C22H36N2O5S/c1-2-3-16-30(27,28)24-21(22(25)26)17-19-7-9-20(10-8-19)29-15-5-4-6-18-11-13-23-14-12-18/h7-10,18,21,23-24H,2-6,11-17H2,1H3,(H,25,26)/t21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tirofiban(L700462;MK383) is a potent non-peptide, glycoprotein IIb/IIIa (integrins alphaIIbbetaIII) antagonist
Target: integrin IIb/IIIa
Tirofiban hydrochloride monohydrate blocks platelet aggregation and thrombus formation. Tirofiban is an antithrombotic used in the treatment of unstable angina.
Tirofiban, in a concentration-dependent manner reduced platelet aggregation evoked by ADP (IC50 approximately 70 ng/ml), collagen (IC50 approximately 200 ng/ml), and thrombin (IC50 approximately 5,000 ng/ml). References: | |||||

Tirofiban Dilution Calculator

Tirofiban Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2696 mL | 11.3482 mL | 22.6963 mL | 45.3926 mL | 56.7408 mL |
| 5 mM | 0.4539 mL | 2.2696 mL | 4.5393 mL | 9.0785 mL | 11.3482 mL |
| 10 mM | 0.227 mL | 1.1348 mL | 2.2696 mL | 4.5393 mL | 5.6741 mL |
| 50 mM | 0.0454 mL | 0.227 mL | 0.4539 mL | 0.9079 mL | 1.1348 mL |
| 100 mM | 0.0227 mL | 0.1135 mL | 0.227 mL | 0.4539 mL | 0.5674 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tirofiban (MK-383) is a selective palate GPIIb/IIIa antagonist which inhibits platelet aggregation with IC50 of 9 nM.
- Clopidogrel Related Compound A
Catalog No.:BCN2687
CAS No.:144457-28-3
- Beta-Aflatrem
Catalog No.:BCN6699
CAS No.:144446-23-1
- Goniodiol 8-acetate
Catalog No.:BCN4787
CAS No.:144429-71-0
- Aflavarin
Catalog No.:BCN7410
CAS No.:144429-67-4
- 3-O-(E)-p-Coumaroylbetulin
Catalog No.:BCN6247
CAS No.:144424-80-6
- Canophyllal
Catalog No.:BCN7441
CAS No.:14440-40-5
- 3-Oxopomolic acid methyl ester
Catalog No.:BCN3723
CAS No.:14440-23-4
- 8-(7-Hydroxy-3,7-dimethyl-2,5-octadienyloxy)psoralen
Catalog No.:BCN1567
CAS No.:144398-34-5
- GSK2838232
Catalog No.:BCC6372
CAS No.:1443461-21-9
- CCG 203971
Catalog No.:BCC5601
CAS No.:1443437-74-8
- Nemoralisin C
Catalog No.:BCN7680
CAS No.:1443421-84-8
- YM 26734
Catalog No.:BCC7396
CAS No.:144337-18-8
- Licochalcone C
Catalog No.:BCN6334
CAS No.:144506-14-9
- Rotundatin
Catalog No.:BCN7856
CAS No.:144506-16-1
- Cannabisin D
Catalog No.:BCN2547
CAS No.:144506-19-4
- Pixantrone
Catalog No.:BCC5222
CAS No.:144510-96-3
- Isorhynchophyllic acid
Catalog No.:BCN4980
CAS No.:144525-05-3
- Oxyresveratrol 3'-O-beta-D-glucopyranoside
Catalog No.:BCN1566
CAS No.:144525-40-6
- Murrayanol
Catalog No.:BCN3178
CAS No.:144525-81-5
- ME0328
Catalog No.:BCC3995
CAS No.:1445251-22-8
- GSK 2837808A
Catalog No.:BCC5607
CAS No.:1445879-21-9
- Kanshone H
Catalog No.:BCN7627
CAS No.:1445952-33-9
- Paliperidone
Catalog No.:BCC3834
CAS No.:144598-75-4
- IRL-1038
Catalog No.:BCC5730
CAS No.:144602-02-8
Validated spectrofluorimetric methods for the determination of apixaban and tirofiban hydrochloride in pharmaceutical formulations.[Pubmed:27984753]
Spectrochim Acta A Mol Biomol Spectrosc. 2017 Mar 5;174:326-330.
Apixaban and Tirofiban Hydrochloride are low molecular weight anticoagulants. The two drugs exhibit native fluorescence that allow the development of simple and valid spectrofluorimetric methods for the determination of Apixaban at lambda ex/lambda em=284/450nm and Tirofiban HCl at lambda ex/lambda em=227/300nm in aqueous media. Different experimental parameters affecting fluorescence intensities were carefully studied and optimized. The fluorescence intensity-concentration plots were linear over the ranges of 0.2-6mugml(-1) for apixaban and 0.2-5mugml(-1) for Tirofiban HCl. The limits of detection were 0.017 and 0.019mugml(-1) and quantification limits were 0.057 and 0.066mugml(-1) for apixaban and Tirofiban HCl, respectively. The fluorescence quantum yield of apixaban and Tirofiban were calculated with values of 0.43 and 0.49. Method validation was evaluated for linearity, specificity, accuracy, precision and robustness as per ICH guidelines. The proposed spectrofluorimetric methods were successfully applied for the determination of apixaban in Eliquis tablets and Tirofiban HCl in Aggrastat intravenous infusion. Tolerance ratio was tested to study the effect of foreign interferences from dosage forms excipients. Using Student's t and F tests, revealed no statistically difference between the developed spectrofluorimetric methods and the comparison methods regarding the accuracy and precision, so can be contributed to the analysis of apixaban and Tirofiban HCl in QC laboratories as an alternative method.
Aspiration thrombectomy and intracoronary tirofiban via GuideLiner((R)) catheter for a thrombosed aneurysmal vessel.[Pubmed:28169555]
Future Cardiol. 2017 Mar;13(2):131-135.
A 52-year-old Asian male with no traditional risk factors for coronary artery disease presented with acute coronary syndrome. Coronary angiography showed complete thrombotic occlusion of the left circumflex with a large thrombus burden in the setting of diffuse aneurysmal enlargement of the coronary arteries consistent with antecedent Kawasaki disease. Manual thrombectomy with adjunctive intracoronary Tirofiban was performed utilizing the GuideLiner catheter((R)) (Vascular Solutions, Inc., MN, USA). Stent implantation was deferred. Follow-up imaging 48 h later showed preserved coronary flow and decreased thrombus burden. The GuideLiner catheter, a monorail guiding device, served a novel role in thrombus aspiration and intracoronary medication delivery.
Assessment of platelet function in patients receiving tirofiban early after primary coronary intervention.[Pubmed:28180001]
Interv Med Appl Sci. 2016 Dec;8(4):135-140.
BACKGROUND: Following percutaneous coronary intervention, combined antiplatelet therapy is necessary. Platelet function testing (PFT) has prognostic value and may be applied in the risk assessment of acute coronary syndrome. In case of combined antiplatelet therapy, PFT may require special laboratory methods, as different antiplatelet agents may influence test results. MATERIALS AND METHODS: Platelet functions were measured in stent thrombosis-segment elevation myocardial infarction patients receiving aspirin, clopidogrel, and Tirofiban. The first sampling was obtained immediately after the termination of administration of Tirofiban. The second sample was drawn at a randomly assigned time between 1 and 6 h. The third sampling was done after a minimum of 24 h of Tirofiban cessation. Adenosine diphosphate (ADP)- and thrombin receptor-activating peptide (TRAP)-induced aggregations were measured. RESULTS: Thirty-seven patients were included. Both TRAP- and ADP-induced aggregation values were significantly lower immediately after Tirofiban termination, than after 24 h [TRAP: 26.41 +/- 25.00 units (U) vs. 109.86 +/- 23.69 U, p < 0.0001; ADP: 17.43 +/- 10.10 U vs. 43.92 +/- 23.35 U, p Tirofiban and clopidogrel were 1.34 +/- 0.49 and 1.269 +/- 0.78, respectively. CONCLUSION: ADP-induced residual platelet reactivity is significantly influenced by the presence of concurrent glycoprotein IIb/IIIa inhibitor. In patients receiving combined antiplatelet treatment, ADP-receptor-specific efficiency measurements are valid only after total elimination of GPIIb/IIIa inhibitors.


