TamarixetinCAS# 603-61-2 |
Quality Control & MSDS
Number of papers citing our products

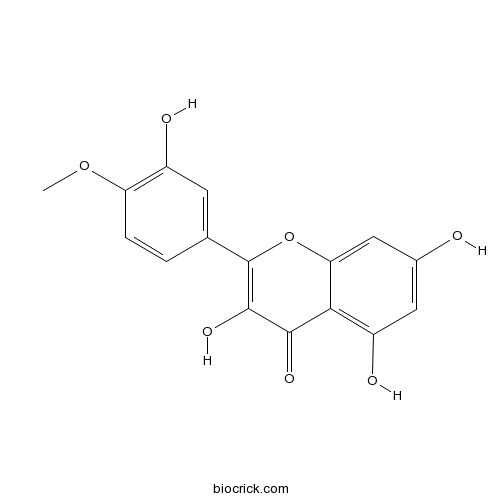
Chemical structure

3D structure
| Cas No. | 603-61-2 | SDF | Download SDF |
| PubChem ID | 5281699 | Appearance | Beige powder |
| Formula | C16H12O7 | M.Wt | 316.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 4'-Methylquercetin; Quercetin 4'-methyl ether; Tamaraxetin; 3,3',5,7-Tetrahydroxy 4'-methoxyflavone | ||
| Solubility | Soluble in DMSO and methanol; insoluble in water | ||
| Chemical Name | 3,5,7-trihydroxy-2-(3-hydroxy-4-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O | ||
| Standard InChIKey | FPLMIPQZHHQWHN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O7/c1-22-11-3-2-7(4-9(11)18)16-15(21)14(20)13-10(19)5-8(17)6-12(13)23-16/h2-6,17-19,21H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Tamarixetin has cytotoxic against leukemia cells and in particular P-glycoprotein- overexpressing K562/ADR cells, it inhibits proliferation in a concentration- and time-dependent manner, induces apoptosis and blocked cell cycle progression at G2 -M phase. 2. Tamarixetin has vasodilator effects in rat isolated vessels. |
| Targets | CDK | p21 | Caspase |

Tamarixetin Dilution Calculator

Tamarixetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1616 mL | 15.8078 mL | 31.6156 mL | 63.2311 mL | 79.0389 mL |
| 5 mM | 0.6323 mL | 3.1616 mL | 6.3231 mL | 12.6462 mL | 15.8078 mL |
| 10 mM | 0.3162 mL | 1.5808 mL | 3.1616 mL | 6.3231 mL | 7.9039 mL |
| 50 mM | 0.0632 mL | 0.3162 mL | 0.6323 mL | 1.2646 mL | 1.5808 mL |
| 100 mM | 0.0316 mL | 0.1581 mL | 0.3162 mL | 0.6323 mL | 0.7904 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chrysosplenetin
Catalog No.:BCN4115
CAS No.:603-56-5
- Auriculasin
Catalog No.:BCN3970
CAS No.:60297-37-2
- Fulvine
Catalog No.:BCN2082
CAS No.:6029-87-4
- Latifoline
Catalog No.:BCN1978
CAS No.:6029-86-3
- Rinderine
Catalog No.:BCN1971
CAS No.:6029-84-1
- 7-Angeloylretronecine
Catalog No.:BCN2036
CAS No.:6029-82-9
- Gestodene
Catalog No.:BCC4490
CAS No.:60282-87-3
- Guvacine hydrochloride
Catalog No.:BCC6574
CAS No.:6027-91-4
- Guanosine-2'(3')-monophosphate disodium salt
Catalog No.:BCC3608
CAS No.:6027-83-4
- Aspalathin
Catalog No.:BCC8122
CAS No.:6027-43-6
- H-D-HoSer-OH
Catalog No.:BCC3243
CAS No.:6027-21-0
- Ethyl(1-hydroxy-4-oxocyclohexa-2,5-dien-1-yl)acetate
Catalog No.:BCN1405
CAS No.:60263-06-1
- Sulforhodamine 101
Catalog No.:BCC8019
CAS No.:60311-02-6
- Odanacatib (MK-0822)
Catalog No.:BCC1197
CAS No.:603139-19-1
- Geissoschizine methyl ether
Catalog No.:BCN7736
CAS No.:60314-89-8
- NBI 35965 hydrochloride
Catalog No.:BCC7567
CAS No.:603151-83-3
- 4-Methoxyphenyl beta-D-glucopyranoside
Catalog No.:BCN1403
CAS No.:6032-32-2
- Sulprostone
Catalog No.:BCC7547
CAS No.:60325-46-4
- GSK-3 inhibitor 1
Catalog No.:BCC4126
CAS No.:603272-51-1
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
- 9-O-Feruloyllariciresinol
Catalog No.:BCN4112
CAS No.:60337-67-9
- Leucovorin Calcium
Catalog No.:BCC1198
CAS No.:6035-45-6
- 6,7,8-Trimethoxycoumarin
Catalog No.:BCN4113
CAS No.:6035-49-0
- Doronine
Catalog No.:BCN2106
CAS No.:60367-00-2
Competition between ascorbate and glutathione for the oxidized form of methylated quercetin metabolites and analogues: tamarixetin, 4'O-methylquercetin, has the lowest thiol reactivity.[Pubmed:22860763]
J Agric Food Chem. 2012 Sep 12;60(36):9292-7.
Quercetin (Q) is a bioactive compound with excellent antioxidant activity. However, the thiol reactivity of its oxidation product (oxQ) forms a disadvantage. The aim of the present study was to decrease this thiol toxicity. We found that methylated Q metabolites displayed lower thiol reactivity than Q. The most effective was Tamarixetin, 4'O-methylquercetin (4'MQ), that has a corresponding oxidation product (ox4'MQ) with thiol reactivity 350 times lower than oxQ. The endogenous metabolism of Q to 4'MQ might be a physiological way to safely benefit from the antioxidant potential of Q in vivo. Our results were explained with Pearson's HSAB concept and corroborated by quantum molecular calculations that revealed a strong correlation between the relative thiol reactivity and the lowest unoccupied molecular orbital (LUMO). The polarity of the molecule and the pi-pi interaction between the AC- and the B-ring appeared to determine the LUMO and the thiol reactivity of the oxidation product.
Induction of G2/M phase arrest and apoptosis by the flavonoid tamarixetin on human leukemia cells.[Pubmed:23765509]
Mol Carcinog. 2014 Dec;53(12):939-50.
Flavonoids are naturally occurring polyphenolic compounds which display a vast array of biological activities. In this study, we investigated the effects of Tamarixetin on viability of human tumor cell lines and found that it was cytotoxic against leukemia cells and in particular P-glycoprotein-overexpressing K562/ADR cells. This compound inhibited proliferation in a concentration- and time-dependent manner, induced apoptosis and blocked cell cycle progression at G2 -M phase. This was associated with the accumulation of cyclin B1, Bub1 and p21(Cip1/Waf-1), changes in the phosphorylation status of cyclin B1, Cdk1, Cdc25C and MPM-2, and inhibition of tubulin polymerization. Moreover, cell death was found to be associated with cytochrome c release and cleavage of caspases and of poly(ADP-ribose) polymerase, and completely abrogated by the free-radical scavenger N-acetyl-L-cysteine. The sensitivity of leukemic cells to Tamarixetin suggests that it should be considered for further preclinical and in vivo testing.
Red wine alcohol promotes quercetin absorption and directs its metabolism towards isorhamnetin and tamarixetin in rat intestine in vitro.[Pubmed:16444288]
Br J Pharmacol. 2006 Apr;147(7):765-71.
Moderate consumption of red wine has been associated with beneficial effects on human health, and this has been attributed to the flavonoid content. Factors that influence the bioavailability of this group of polyphenolic compounds are therefore important. Using the rat cannulated everted jejunal sac technique, we have investigated the effect of alcohol on the intestinal absorption of quercetin and its 3-O-glucoside from red wine. Tissue preparations were incubated in whole or dealcoholised red wine, diluted 1 : 1 with Krebs buffer for 20 min at 37 degrees C, after which the mucosa was removed and processed for HPLC analysis. Tissues exposed to red wine had significantly higher amounts of both quercetin (x 3; P < 0.001) and quercetin-3-O-glucoside (x 1.5; P < 0.01) associated with them, compared with sacs incubated in the dealcoholised equivalent. In addition, both Tamarixetin (T) and isorhamnetin (I), in the mucosal tissue from sacs exposed to the whole wine, were significantly elevated approximately two fold (P < 0.05; P < 0.01, respectively). Similar results were obtained when sacs were incubated in Krebs buffer containing a mixture of pure quercetin and quercetin-3-O-glucoside with or without alcohol, and, although effects on the apparent absorption of Q and Q-3-G were not so marked, concentrations of the metabolites quercetin-3-O-glucuronide and I were significantly increased by the presence of alcohol (P < 0.01 and P < 0.001, respectively). It is therefore plausible that the moderate alcohol content of red wine contributes to its beneficial health effects in humans by both increasing the absorption of quercetin and quercetin-3-O-glucoside and by channelling their metabolism towards O-methylation to yield compounds (T and I), which have potential protective effects against cancer and cardiovascular diseases.


