TSTUCAS# 105832-38-0 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
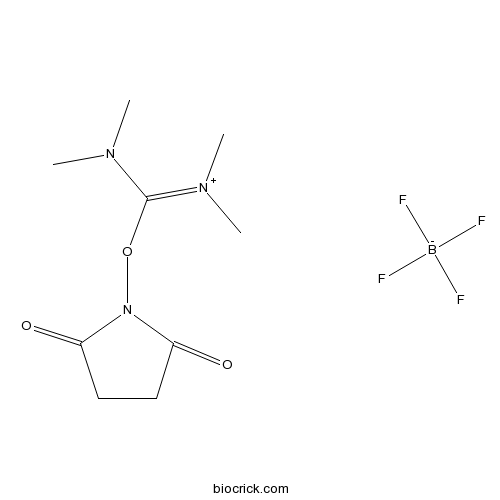
| Cas No. | 105832-38-0 | SDF | Download SDF |
| PubChem ID | 9857522 | Appearance | Powder |
| Formula | C9H16BF4N3O3 | M.Wt | 301.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in water or 1% acetic acid | ||
| Chemical Name | [dimethylamino-(2,5-dioxopyrrolidin-1-yl)oxymethylidene]-dimethylazanium;tetrafluoroborate | ||
| SMILES | [B-](F)(F)(F)F.CN(C)C(=[N+](C)C)ON1C(=O)CCC1=O | ||
| Standard InChIKey | YEBLHMRPZHNTEK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H16N3O3.BF4/c1-10(2)9(11(3)4)15-12-7(13)5-6-8(12)14;2-1(3,4)5/h5-6H2,1-4H3;/q+1;-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

TSTU Dilution Calculator

TSTU Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3212 mL | 16.6058 mL | 33.2116 mL | 66.4231 mL | 83.0289 mL |
| 5 mM | 0.6642 mL | 3.3212 mL | 6.6423 mL | 13.2846 mL | 16.6058 mL |
| 10 mM | 0.3321 mL | 1.6606 mL | 3.3212 mL | 6.6423 mL | 8.3029 mL |
| 50 mM | 0.0664 mL | 0.3321 mL | 0.6642 mL | 1.3285 mL | 1.6606 mL |
| 100 mM | 0.0332 mL | 0.1661 mL | 0.3321 mL | 0.6642 mL | 0.8303 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TSTU
- Tropisetron Hydrochloride
Catalog No.:BCC4027
CAS No.:105826-92-4
- Nateglinide
Catalog No.:BCC5005
CAS No.:105816-04-4
- E-3810
Catalog No.:BCC1541
CAS No.:1058137-23-7
- Sitostenone
Catalog No.:BCN5868
CAS No.:1058-61-3
- Fmoc-Ser(tBu)-OPfp
Catalog No.:BCC3545
CAS No.:105751-13-1
- Methyl ganoderate C6
Catalog No.:BCN3259
CAS No.:105742-81-2
- Ganoderic acid C6
Catalog No.:BCN3257
CAS No.:105742-76-5
- AL 8697
Catalog No.:BCC8037
CAS No.:1057394-06-5
- Androstanolone 17-benzoate
Catalog No.:BCC8825
CAS No.:1057-07-4
- AT13148
Catalog No.:BCC5360
CAS No.:1056901-62-2
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- CYT387
Catalog No.:BCC2196
CAS No.:1056634-68-4
- Obtusilin
Catalog No.:BCN2697
CAS No.:105870-59-5
- (tert-Butoxycarbonyl)oxycefcapene pivoxil
Catalog No.:BCC8403
CAS No.:105889-80-3
- Taraxasterol
Catalog No.:BCN5869
CAS No.:1059-14-9
- Doxycycline HCl
Catalog No.:BCC3772
CAS No.:10592-13-9
- STEARDA
Catalog No.:BCC7288
CAS No.:105955-10-0
- OLDA
Catalog No.:BCC7138
CAS No.:105955-11-1
- Clinafloxacin CI96 AM1091
Catalog No.:BCC3754
CAS No.:105956-97-6
- Sulfocostunolide B
Catalog No.:BCN5870
CAS No.:1059671-65-6
- 2-(3,4-Dihydroxyphenyl)ethanol
Catalog No.:BCN5871
CAS No.:10597-60-1
- Geraniol
Catalog No.:BCN2631
CAS No.:106-24-1
- Nerol
Catalog No.:BCN8517
CAS No.:106-25-2
- β-Interleukin I (163-171), human
Catalog No.:BCC1017
CAS No.:106021-96-9
Synthesis and solid state NMR characterization of novel peptide/silica hybrid materials.[Pubmed:26411982]
Solid State Nucl Magn Reson. 2015 Nov;72:73-8.
The successful synthesis and solid state NMR characterization of silica-based organic-inorganic hybrid materials is presented. For this, collagen-like peptides are immobilized on carboxylate functionalized mesoporous silica (COOH/SiOx) materials. A pre-activation of the silica material with TSTU (O-(N-Succinimidyl)-N,N,N',N'-tetramethyluronium tetrafluoroborate) is performed to enable a covalent binding of the peptides to the linker. The success of the covalent immobilization is indicated by the decrease of the (13)C CP-MAS NMR signal of the TSTU moiety. A qualitative distinction between covalently bound and adsorbed peptide is feasible by (15)N CP-MAS Dynamic Nuclear Polarization (DNP). The low-field shift of the (15)N signal of the peptide's N-terminus clearly identifies it as the binding site. The DNP enhancement allows the probing of natural abundance (15)N nuclei, rendering expensive labeling of peptides unnecessary.
Seasonal incidence of lameness and risk factors associated with thin soles, white line disease, ulcers, and sole punctures in dairy cattle.[Pubmed:19528594]
J Dairy Sci. 2009 Jul;92(7):3165-74.
Lameness is a multifactorial condition with many causes. In this study, cow lifetime records were used to quantify the incidence of specific lameness-causing lesions and investigate factors associated with those lesions. Of primary interest were the effects of seasonality and the effects of thin soles (TS). Thin sole-induced toe ulcers (TSTU) occurring adjacent to the white line in the apical portion of the weight-bearing surface were distinguished from white line disease (WLD) occurring in the region of the abaxial heel sole junction. Sole (SU), heel (HU), and toe (TU) ulcers; TS; sole punctures (SP); leg injuries (INJ); and other (OTH) lesions (e.g., infectious diseases, laminitis, unclassified hemorrhage) were also considered. Data were collected from May 2004 through October 2007 and included records for 4,915 cows of which 1,861 had at least one recorded lameness event. Of these, 20% were TSTU, 20% OTH, 16% SU, 13% TS, 10% WLD, 8% HU, 6% INJ, 4% SP, and 2% TU. Annual incidence risk for lameness was 49.1%. Overall incidence rate for lameness was 1.41/1,000 cow-days, and rates for all lesions were highest in the summer. As parity increased, so did incidence rates for TS, SU, WLD, HU, and INJ. For TS, TSTU, and WLD, incidence rates were lowest in early lactation (16 to 60 DIM), whereas for SU, HU, TU, incidence rates were highest in mid lactation (61 to 150 DIM). Cox proportional hazard models for TS, TSTU, WLD, SU, HU, TU, and SP included age and year of first calving and milk production capacity. Prior/concurrent lameness events, season, parity, and stage of lactation were included as time-dependent effects. Prior/concurrent TS increased the hazard for all other lesions, particularly TSTU, and HU. Having any other prior claw lesion also increased the hazard for all lesions. Hazard was highest in summer for all lesions except TU. Stage of lactation was a significant effect in hazard of TSTU, which was lowest in mid lactation (61 to 150 DIM).
Optimization of a convenient route to produce N-succinimidyl 4-radiodobenzoate for radioiodination of proteins.[Pubmed:12798375]
Appl Radiat Isot. 2003 Jun;58(6):667-73.
The preparation of N-succinimidyl-4-[131I]iodobenzoate (SIB) has been optimized using an alternative technique employing Cu(I)-assisted radioiododebromination that produces p-[131I]iodobenzoic acid. The reaction conditions were optimized and radiochemical purity of more than 90% was obtained when using 160 degrees C, 60 min reaction time and a [CuCl]/[p-bromobenzoic acid] relation of about 10(-2). After purification, the p-[131I]iodobenzoic acid reacted with TSTU to produce the SIB in a radiochemical yield greater than 98%. Protein conjugation using SIB resulted in a relatively low radiochemical yield. Biological distribution studies evidenced the in vivo stability of the labeled protein.
Photostable, amino reactive and water-soluble fluorescent labels based on sulfonated rhodamine with a rigidized xanthene fragment.[Pubmed:18058955]
Chemistry. 2008;14(6):1784-92.
Highly water soluble fluorescent dyes were synthesized and transformed into new amino reactive fluorescent labels for biological microscopy. To this end, rhodamine 8 (prepared from 7-hydroxy-1,2,3,4-tetrahydroquinoline (7) and phthalic anhydride in 85 % aq. H(3)PO(4)) was sulfonated with 30 % SO(3) in H(2)SO(4) and afforded the water soluble disulfonic acid 3 a (64 %). Amidation of the carboxy group in 3 a with 2-(methylamino)ethanol in the presence of O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluroniumPF(6) (-) (HATU) led to alcohol 3 b (66 %), which was transformed into the amino reactive mixed carbonate 3 d with di(N-succinimidyl)carbonate and Et(3)N. Reaction of the carboxy group in 3 a with MeNH(CH(2))(2)CO(2)Me and N,N,N',N'-tetramethyl-O-(N-succinimidyl)-uroniumBF(4) (-) (TSTU) yielded methyl ester 13. After saponification of the aliphatic carboxy group in 13, the compound was converted into NHS-ester 3 e (using HATU and Et(3)N). Heating of 7 with trimellitic anhydride in H(3)PO(4) gave a mixture of dicarboxylic acids 14 and 15 (1:1). Regioisomer 15 was isolated, sulfonated with 30 % SO(3) in H(2)SO(4), and disulfonic acid 3 f was used for the synthesis of the mono NHS-ester 3 g, in which the sterically unhindered carboxy group was selectively activated (with N-hydroxysuccinimide, HATU, and Et(3)N). The sulfonated rhodamines 3 b, c and f are soluble in water (up to 0.1 M), have excellent photostabilities and large fluorescence quantum yields. Subdiffraction resolution images of tubulin filaments of mammalian cells stained with these dyes illustrate their applicability as labels for stimulated emission depletion microscopy and other fluorescence techniques.
Radioiodination of proteins using prosthetic group: a convenient way to produce labelled proteins with in vivo stability.[Pubmed:12619967]
Cell Mol Biol (Noisy-le-grand). 2002 Nov;48(7):735-9.
Radiolabelled peptides can provide new approaches for radiopharmaceutical development. Several prosthetic groups have been developed for radioiodination of proteins in order to minimize in vivo dehalogenation. In this work, the prosthetic group N-succinimidyl 4-[131I]iodobenzoate ([131I]SIB) was obtained by an alternative procedure that employs Cu(I) assisted radioiododebromination to produce p-[131I]iodobenzoic acid with a radiochemical yield of 92.73 +/- 1.51% (N = 6), followed by the reaction with TSTU (O-(N-succinimidyl)-N,N,N'N'-tetramethyluronium) in alkaline medium. The HPLC profile of the final product, revealed that [131I]SIB was obtained with a radiochemical purity of 98.19 +/- 1.14% (N = 6 Swiss mices (normal group) and animals with inflammation focus developed on the right thigh by tupertine injection) were injected with human immunoglobulin (IgG) radioiodinated with [131I]SIB and by direct method (Iodogen). The comparison of results showed a fast blood clearance, better target organ/background relation and low uptake in thyroid and stomach (p < 0.01) for the protein labelled with [131I]SIB, what suggests a greater in vivo stability.


