Senkyunolide ACAS# 62006-39-7 |
- Senkyunolide
Catalog No.:BCN8154
CAS No.:63038-10-8
Quality Control & MSDS
Number of papers citing our products

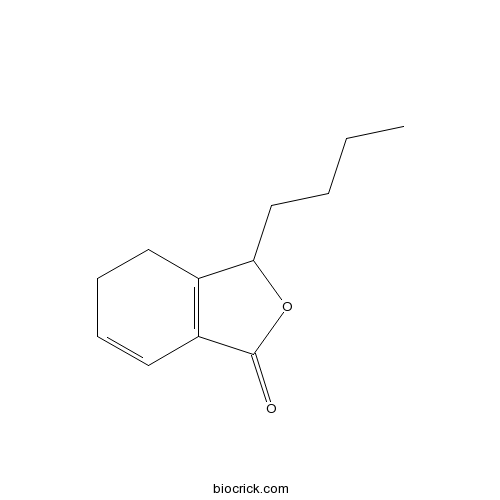
Chemical structure

3D structure
| Cas No. | 62006-39-7 | SDF | Download SDF |
| PubChem ID | 173843 | Appearance | Oil |
| Formula | C12H16O2 | M.Wt | 192.25 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-butyl-4,5-dihydro-3H-2-benzofuran-1-one | ||
| SMILES | CCCCC1C2=C(C=CCC2)C(=O)O1 | ||
| Standard InChIKey | ZPIKVDODKLJKIN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H16O2/c1-2-3-8-11-9-6-4-5-7-10(9)12(13)14-11/h5,7,11H,2-4,6,8H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Senkyunolide A is a useful standard compound for the quality evaluation and chemical differentiation between Rhizoma chuanxiong and Angelica sinensis, and suitable for the analysis of a large number of samples. Senkyunolide A has the vasorelaxation activity in contractions to various contractile agents in rat isolated aorta. |
| In vitro | Relaxation effects of ligustilide and senkyunolide A, two main constituents of Ligusticum chuanxiong, in rat isolated aorta.[Pubmed: 17222996]J Ethnopharmacol. 2007 May 22;111(3):677-80.Ligusticum chuanxiong Hort. (Umbelliferae) is a widely prescribed traditional Chinese medicinal herb for cardiovascular diseases in China. However, the cardiovascular actions of ligustilide and Senkyunolide A, two of the most abundant Ligusticum chuanxiong constituents, have yet to be examined. The objective of the present study was to investigate the vasorelaxation effects of ligustilide and Senkyunolide A and their underlying mechanisms in rat isolated aorta. |
| In vivo | Low oral bioavailability and pharmacokinetics of senkyunolide a, a major bioactive component in Rhizoma Chuanxiong, in the rat.[Pubmed: 17304150]Ther Drug Monit. 2007 Feb;29(1):49-56.The pharmacokinetics of Senkyunolide A, one of the major bioactive ingredients in the traditional Chinese medicinal herb Rhizoma Chuanxiong, which is commonly used for the treatment of cardiovascular diseases, was studied in rats. |
| Structure Identification | Zhongguo Zhong Yao Za Zhi. 2014 May;39(9):1650-5.[Quantitative determination of 5 active ingredients in different harvest periods of Ligusticum chuanxiong by HPLC].[Pubmed: 25095378]A simple and quick method is described for the determination of ferulic acid, senkyunolide I, senkyunolide H, Senkyunolide A and ligustilide in rhizomes of Ligusticum chuanxiong. Chem Pharm Bull (Tokyo). 2005 Nov;53(11):1480-3.Identification and comparative determination of senkyunolide A in traditional Chinese medicinal plants Ligusticum chuanxiong and Angelica sinensis by HPLC coupled with DAD and ESI-MS.[Pubmed: 16272738]
|

Senkyunolide A Dilution Calculator

Senkyunolide A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2016 mL | 26.0078 mL | 52.0156 mL | 104.0312 mL | 130.039 mL |
| 5 mM | 1.0403 mL | 5.2016 mL | 10.4031 mL | 20.8062 mL | 26.0078 mL |
| 10 mM | 0.5202 mL | 2.6008 mL | 5.2016 mL | 10.4031 mL | 13.0039 mL |
| 50 mM | 0.104 mL | 0.5202 mL | 1.0403 mL | 2.0806 mL | 2.6008 mL |
| 100 mM | 0.052 mL | 0.2601 mL | 0.5202 mL | 1.0403 mL | 1.3004 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4,4'-Methylenediphenol
Catalog No.:BCN2690
CAS No.:620-92-8
- Hyoscyamine sulfate hydrate
Catalog No.:BCN2846
CAS No.:620-61-1
- Tribenzylamine
Catalog No.:BCN1817
CAS No.:620-40-6
- 5-Methyl-2-furaldehyde
Catalog No.:BCN3801
CAS No.:620-02-0
- Diphemanil Methylsulfate
Catalog No.:BCC3767
CAS No.:62-97-5
- Nandrolone phenylpropionate
Catalog No.:BCC9089
CAS No.:62-90-8
- Cevadine acetate
Catalog No.:BCC8144
CAS No.:63982-55-8
- H-Aib-OH
Catalog No.:BCC3207
CAS No.:62-57-7
- Lipoic acid
Catalog No.:BCN5980
CAS No.:62-46-4
- Phenacetin
Catalog No.:BCC4436
CAS No.:62-44-2
- Dopamine hydrochloride
Catalog No.:BCN2195
CAS No.:62-31-7
- Adrenalone HCl
Catalog No.:BCC4323
CAS No.:62-13-5
- CCG-63802
Catalog No.:BCC1460
CAS No.:620112-78-9
- CCG-63808
Catalog No.:BCC1461
CAS No.:620113-73-7
- Dirithromycin
Catalog No.:BCC4656
CAS No.:62013-04-1
- p-Menthane-1,2,8-triol
Catalog No.:BCN4148
CAS No.:62014-81-7
- Helichrysetin
Catalog No.:BCN4149
CAS No.:62014-87-3
- Bulgarsenine
Catalog No.:BCN2065
CAS No.:62018-77-3
- Cyclobenzaprine HCl
Catalog No.:BCC6496
CAS No.:6202-23-9
- Juncusol
Catalog No.:BCN2926
CAS No.:62023-90-9
- Ginsenoside F2
Catalog No.:BCN1245
CAS No.:62025-49-4
- Ginsenoside F3
Catalog No.:BCN1077
CAS No.:62025-50-7
- 4-(Dimethylamino)cinnamaldehyde
Catalog No.:BCN4968
CAS No.:6203-18-5
- Onitisin 2'-O-glucoside
Catalog No.:BCN4150
CAS No.:62043-53-2
Relaxation effects of ligustilide and senkyunolide A, two main constituents of Ligusticum chuanxiong, in rat isolated aorta.[Pubmed:17222996]
J Ethnopharmacol. 2007 May 22;111(3):677-80.
Ligusticum chuanxiong Hort. (Umbelliferae) is a widely prescribed traditional Chinese medicinal herb for cardiovascular diseases in China. However, the cardiovascular actions of ligustilide and Senkyunolide A, two of the most abundant Ligusticum chuanxiong constituents, have yet to be examined. The objective of the present study was to investigate the vasorelaxation effects of ligustilide and Senkyunolide A and their underlying mechanisms in rat isolated aorta. Both constituents had similar relaxation potencies against contractions to 9,11-dideoxy-9alpha,11alpha-methanoepoxyprostaglandin F(2alpha), phenylephrine, 5-hydroxytryptamine and KCl. Their vasorelaxation effects were not affected by endothelium removal, the adenylate cyclase inhibitor 9-(tetrahydro-2-furanyl)-9H-purin-6-amine, the soluble guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, or the non-selective K+ channel blocker tetraethylammonium. This is the first report to demonstrate the vasorelaxation activities of ligustilide and Senkyunolide A in contractions to various contractile agents in rat isolated aorta. The underlying mechanisms await further investigations.
Identification and comparative determination of senkyunolide A in traditional Chinese medicinal plants Ligusticum chuanxiong and Angelica sinensis by HPLC coupled with DAD and ESI-MS.[Pubmed:16272738]
Chem Pharm Bull (Tokyo). 2005 Nov;53(11):1480-3.
Using the HPLC/DAD/ESI/MS method, the qualitative and quantitative analysis of Senkyunolide A (SA) in the rhizomes of Ligusticum chuanxiong (Rhizoma chuanxiong; CX) and roots of Angelica sinensis (DG) was established. As a result, it was found that SA is a characteristic standard compound for the quality evaluation and chemical differentiation between CX and DG. Methanol was chosen in the preparation of standard solutions and extraction of samples based on the stability data. The identity of SA in CX and DG was unambiguously determined based on the quasimolecular ions in ESI-MS. A comprehensive validation of the method, including sensitivity, linearity, reproducibility and recovery, was conducted using the optimized chromatographic conditions. The linear calibration curve was acquired with R2>0.999 and limit of detection (S/N=3) was estimated to be 12.5 mug/g. The reproducibility was evaluated by repeated sample injection and replicated analysis of samples with the relative standard deviation (RSD) value found within 0.68%. The recovery rates of SA varied within the range of 96.91-101.50% with RSD less than 2.38%. In the present work, the contents of SA were quantified within 3.94-9.14 mg/g and 0.108-0.588 mg/g for 12 batches each of CX and DG. The results demonstrated that SA is a useful standard compound for the quality evaluation and chemical differentiation between CX and DG. The analytical procedure is precise and reproducible and thus suitable for the analysis of a large number of samples.
[Quantitative determination of 5 active ingredients in different harvest periods of Ligusticum chuanxiong by HPLC].[Pubmed:25095378]
Zhongguo Zhong Yao Za Zhi. 2014 May;39(9):1650-5.
A simple and quick method is described for the determination of ferulic acid, senkyunolide I, senkyunolide H, Senkyunolide A and ligustilide in rhizomes of Ligusticum chuanxiong. The 5 active ingredients in the sample was extracted using 40% ethanol and analyzed by reversed-phase high performance liquid chromatography (HPLC). Chromatography separation was performed using Agilent 1100 series HPLC system with a Symmetry C18 column and gradient elution with a mixture of three solvents : solvent A, acetonitrile, solvent B, methanol and solvent C, 1% aqueous acetic acid, 0 min to 5 min A: B: C 20: 40: 40, 5 min to 30 min A: B: C 60 to 100 : 0 : 40 to 0. The effluent was monitored using a VWD detector set at 321 nm (0-4.3 min) and 275 nm (4.31-30 min). The flow rate was set at 1 mL x min(-1) and the injection volume was 10 microL. The column temperature was maintained at 35 degrees C. The calibration curve was linear (r > or = 0.99) over the tested ranges. The average recovery was 94.44%-103.1% (n = 6). The method has been successfully applied to the analysis in different harvest periods of L. chuanxiong samples. In this paper, single-factor randomized block design to study the 5 components content of L. chuanxiong on ten collecting stages. For the L. chuanxiong collected from April 15th to May 30rd, the content of 5 ingredients increased primarily, and then decreased. Determine the appropriate harvest time has important significance to the promotion of the quality of L. chuanxiong.
Low oral bioavailability and pharmacokinetics of senkyunolide a, a major bioactive component in Rhizoma Chuanxiong, in the rat.[Pubmed:17304150]
Ther Drug Monit. 2007 Feb;29(1):49-56.
The pharmacokinetics of Senkyunolide A, one of the major bioactive ingredients in the traditional Chinese medicinal herb Rhizoma Chuanxiong, which is commonly used for the treatment of cardiovascular diseases, was studied in rats. After intravenous (IV) administration, Senkyunolide A was extensively distributed (Vd/F: 6.74 +/- 0.73 L/kg) and rapidly eliminated from the plasma (CL/F: 7.20 +/- 0.48 L/h per kilogram and t1/2: 0.65 +/- 0.06 hr). Hepatic metabolism was suggested as the major route of Senkyunolide A elimination as indicated by the results of in vitro S9 fraction study. After intraperitoneal (IP) administration, Senkyunolide A exhibited dose-independent pharmacokinetics. The absorption after IP administration was rapid (Tmax: 0.04 +/- 0.01 hours), and the bioavailability was 75%. After oral administration, Senkyunolide A was also absorbed rapidly (Tmax: 0.21 +/- 0.08 hours); however, its oral bioavailability was low (approximately 8%). The contributing factors were determined to be instability in the gastrointestinal tract (accounting for 67% of the loss) and hepatic first-pass metabolism (accounting for another 25%). Pharmacokinetics of Senkyunolide A were unaltered when Chuanxiong extract was administered, which suggests that components in the extract have insignificant effects on Senkyunolide A pharmacokinetics.


