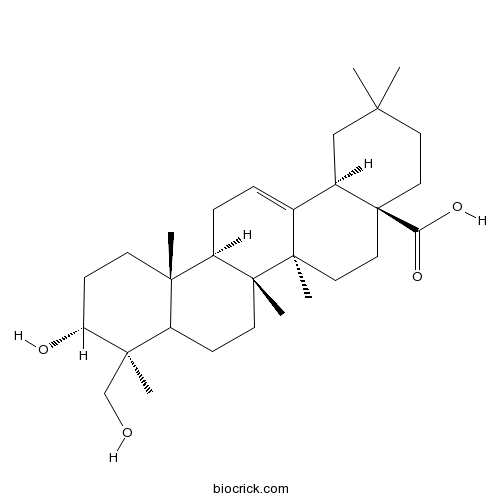
Scutellaric acidCAS# 102919-76-6 |
- Wilforol C
Catalog No.:BCN1100
CAS No.:168254-95-3
- Hederagenin
Catalog No.:BCN5513
CAS No.:465-99-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 102919-76-6 | SDF | Download SDF |
| PubChem ID | 190461 | Appearance | Powder |
| Formula | C30H48O4 | M.Wt | 472.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4aS,6aR,6aS,6bR,9S,10R,12aR,14bR)-10-hydroxy-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | ||
| SMILES | CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)CO)O)C)C)C2C1)C)C(=O)O)C | ||
| Standard InChIKey | PGOYMURMZNDHNS-XXIVSGBYSA-N | ||
| Standard InChI | InChI=1S/C30H48O4/c1-25(2)13-15-30(24(33)34)16-14-28(5)19(20(30)17-25)7-8-22-26(3)11-10-23(32)27(4,18-31)21(26)9-12-29(22,28)6/h7,20-23,31-32H,8-18H2,1-6H3,(H,33,34)/t20-,21?,22-,23-,26+,27-,28-,29-,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Scutellaric acid Dilution Calculator

Scutellaric acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1155 mL | 10.5775 mL | 21.1551 mL | 42.3101 mL | 52.8877 mL |
| 5 mM | 0.4231 mL | 2.1155 mL | 4.231 mL | 8.462 mL | 10.5775 mL |
| 10 mM | 0.2116 mL | 1.0578 mL | 2.1155 mL | 4.231 mL | 5.2888 mL |
| 50 mM | 0.0423 mL | 0.2116 mL | 0.4231 mL | 0.8462 mL | 1.0578 mL |
| 100 mM | 0.0212 mL | 0.1058 mL | 0.2116 mL | 0.4231 mL | 0.5289 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MDL 73005EF hydrochloride
Catalog No.:BCC6636
CAS No.:102908-60-1
- Pexidartinib (PLX3397)
Catalog No.:BCC6405
CAS No.:1029044-16-3
- 17-Hydroxy sprengerinin C
Catalog No.:BCN2755
CAS No.:1029017-75-1
- Lycopsamine
Catalog No.:BCN1999
CAS No.:10285-07-1
- Intermedine
Catalog No.:BCN1997
CAS No.:10285-06-0
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- Ganolactone B
Catalog No.:BCN2872
CAS No.:1028449-53-7
- Moracin P
Catalog No.:BCN3289
CAS No.:102841-46-3
- Mulberroside C
Catalog No.:BCN6344
CAS No.:102841-43-0
- Mulberroside A
Catalog No.:BCN6343
CAS No.:102841-42-9
- D-Pinitol
Catalog No.:BCN5842
CAS No.:10284-63-6
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- INCB28060
Catalog No.:BCC3793
CAS No.:1029712-80-8
- Trelagliptin succinate
Catalog No.:BCC2015
CAS No.:1029877-94-8
- H-DL-Phg-OH
Catalog No.:BCC3317
CAS No.:103-01-5
- Monobenzone
Catalog No.:BCC3818
CAS No.:103-16-2
- Methyl cinnamate
Catalog No.:BCN5043
CAS No.:103-26-4
- Ethyl cinnamate
Catalog No.:BCN5044
CAS No.:103-36-6
- Benzyl cinnamate
Catalog No.:BCN5042
CAS No.:103-41-3
- Scutebarbatine M
Catalog No.:BCN8327
CAS No.:960302-92-5
- N-Methylbenzylamine
Catalog No.:BCN1790
CAS No.:103-67-3
- Phenylacetic Acid
Catalog No.:BCC8349
CAS No.:103-82-2
- 4'-Methylacetanilide
Catalog No.:BCC8714
CAS No.:103-89-9
- Acetaminophen
Catalog No.:BCC5269
CAS No.:103-90-2
New phenolics from the root of Scutellaria prostrata JACQ. ex BENTH.[Pubmed:25040064]
Nat Prod Res. 2014;28(20):1685-90.
Scutellaria prostrata (Lamiaceae), a perennial herb growing as a lonely species in Kashmir, Himalayas, was subjected to repetitive column and flash chromatographic isolation for its chemical documentation-cum-bioevaluation. The methanolic extract of S.prostrata afforded the isolation of ten compounds (1-10), including two new compounds - scutellapbiflavanone (1) and scutellaprostin M (2). The known compounds were found to be scutellarin (3), hispidulin-7-O-beta-D-glucopyranoside (4), baicalin (5), wogonoside (6), scutellaprostin C (7), acetoside (8), martynoside (9) and Scutellaric acid-3-O-beta-D-glucopyranoside (10). Isolation of biflavonoids, phenolics and phenylethanoid compounds from S. prostrata seals a deal of chemotaxonomic importance of this particular species. The characterisation of the compounds was achieved by (1)H, (13)C, (1)H-(1)H DFQ COSY, HMBC, HSQC, HMQC and ROESY NMR experiments. All the compounds were tested for antioxidant, antimicrobial and cytotoxic activities.
Isolation and absolute stereochemistry of coussaric acid, a new bioactive triterpenoid from the stems of Coussarea brevicaulis.[Pubmed:12946428]
Phytochemistry. 2003 Sep;64(1):293-302.
Coussaric acid (1), a triterpenoid based on an ursane skeleton, and an oleanane-type triterpene acid, 3-epi-spathodic acid (2), as well as four known compounds, barbinervic acid, Scutellaric acid, stigmasterol and stigmasterol glucoside, have been isolated from an EtOAc-soluble extract of the stems of Coussarea brevicaulis. The structures of compounds 1 and 2 were elucidated on the basis of spectroscopic investigation, and single-crystal X-ray crystallography was used to confirm the structure of 1. The absolute stereochemistry of 1 was established by chemical transformations and by the Mosher ester procedure. The potential of the isolates and chemical transformation products to induce quinone reductase was evaluated in mouse Hepa lclc7 hepatoma cells.
Scuteflorins A and B, dihydropyranocoumarins from Scutellaria lateriflora.[Pubmed:19555121]
J Nat Prod. 2009 Jun;72(6):983-7.
Two new dihydropyranocoumarins, scuteflorins A (1) and B (2), together with the known compounds decursin (3), chrysin (4), oroxylin A (5), wogonin (6), 5,7-dihydroxy-8,2'-dimethoxyflavone, dihydrochrysin, dihydrooroxylin A, lupenol, Scutellaric acid, pomolic acid, ursolic acid, beta-sitosterol, daucosterol, and palmitic acid, were isolated from the aerial parts of Scutellaria lateriflora, commonly used as a dietary supplement. The structures of 1 and 2 were established by means of 1D and 2D NMR spectra as well as HRMS data. The absolute configuration of coumarins 1 and 2 was determined by comparison of experimental and theoretical calculated CD spectra. The cytotoxicity and antioxidant effects of the methanol extract of this plant and some of the constituent flavonoids were evaluated in vitro.


