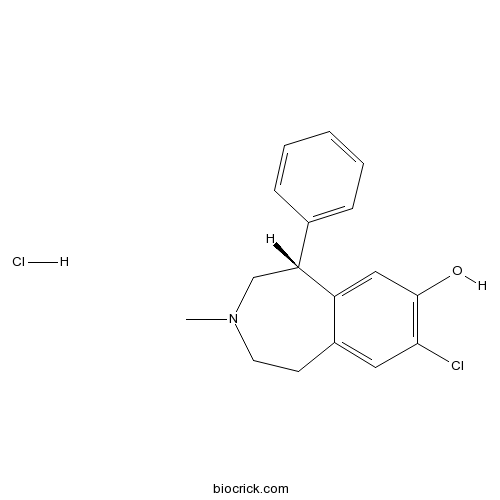
SCH 23390 hydrochloride5-HT2C agonist; also potent dopamine D1-like antagonist, 5-HT1C agonist and Kir3 channel blocker CAS# 125941-87-9 |
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- VX-680 (MK-0457,Tozasertib)
Catalog No.:BCC2167
CAS No.:639089-54-6
- Reversine
Catalog No.:BCC1892
CAS No.:656820-32-5
- AZD1152
Catalog No.:BCC1393
CAS No.:722543-31-9
- XL228
Catalog No.:BCC2058
CAS No.:898280-07-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 125941-87-9 | SDF | Download SDF |
| PubChem ID | 11957535 | Appearance | Powder |
| Formula | C17H19Cl2NO | M.Wt | 324.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (<em>R</em>)-(+)-SCH 23390 hydrochloride | ||
| Solubility | DMSO : ≥ 32 mg/mL (98.69 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (5R)-8-chloro-3-methyl-5-phenyl-1,2,4,5-tetrahydro-3-benzazepin-7-ol;hydrochloride | ||
| SMILES | CN1CCC2=CC(=C(C=C2C(C1)C3=CC=CC=C3)O)Cl.Cl | ||
| Standard InChIKey | OYCAEWMSOPMASE-XFULWGLBSA-N | ||
| Standard InChI | InChI=1S/C17H18ClNO.ClH/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12;/h2-6,9-10,15,20H,7-8,11H2,1H3;1H/t15-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent dopamine receptor antagonist (Ki values are 0.2 nM and 0.3 nM at D1 and D5 receptor sub-types, respectively). Also an agonist at 5-HT1C and 5-HT2C receptors in vitro (Ki values are 6.3 nM and 9.3 nM respectively). Blocks quinpirole-induced Kir3 (GIRK) currents (EC50 = 268 nM) independently of receptors. |

SCH 23390 hydrochloride Dilution Calculator

SCH 23390 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0841 mL | 15.4207 mL | 30.8414 mL | 61.6827 mL | 77.1034 mL |
| 5 mM | 0.6168 mL | 3.0841 mL | 6.1683 mL | 12.3365 mL | 15.4207 mL |
| 10 mM | 0.3084 mL | 1.5421 mL | 3.0841 mL | 6.1683 mL | 7.7103 mL |
| 50 mM | 0.0617 mL | 0.3084 mL | 0.6168 mL | 1.2337 mL | 1.5421 mL |
| 100 mM | 0.0308 mL | 0.1542 mL | 0.3084 mL | 0.6168 mL | 0.771 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SCH 23390 hydrochloride is a potent dopamine receptor D1 antagonist with Ki values of 0.2 and 0.3 nM for the D1 and D5.
In Vitro:The commercially available hydrochloride salt has a molecular weight of 324.25 and is a white solid soluble in water (8 mg/mL), DMSO (3 mg/mL), or ethanol (2 mg/mL)[1]. SCH 23390 also shows high affinity (Ki=9.3 nM) at h5-HT2C sites[2]. SCH23390 blocks endogenous GIRK currents induced by either somatostatin or D3 dopamine receptors in AtT-20 cells (IC50=268 nM)[3].
In Vivo:SCH 23390 has been a major tool in gaining a better understanding of the role of the dopamine system. SCH 23390 is a very short-acting compound with an elimination half-life of around 25 min following administration of 0.3 mg/kg i.p. in the rat[1]. The repeated administration of SCH 23390 (0.05 mg/kg s.c., thrice daily for 21 days) enhances the steady-state density of dopamine D1 receptors in the striatum (+30%) and substantia nigra (+24%). This treatment also increases the production rates of dopamine D1 receptors in the striatum (+44%) and substantia nigra (+54%)[4]. Systemic SCH 23390 reduces saccharin seeking evidenced by a significant reduction in active lever responding and a significant reduction in the number of active lever-contingent deliveries of the tone + light cue following pretreatment with 10 μg/kg SCH 23390[5].
References:
[1]. Bourne JA, et al . SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS Drug Rev. 2001 Winter 7(4):399-414.
[2]. Millan MJ, et al. The selective dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacology (Berl). 2001 Jun 156(1):58-62.
[3]. Kuzhikandathil EV, et al. Classic D1 dopamine receptor antagonist R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) directly inhibits G protein-coupledinwardly rectifying potassium channels. Mol Pharmacol. 2002 Jul 62(1):119-26.
[4]. Giorgi O, et al. Chronic treatment with SCH 23390 increases the production rate of dopamine D1 receptors in the nigro-striatal system of the rat. Eur J Pharmacol. 1993 Apr 15 245(2):139-45.
[5]. Aoyama K, et al. Systemic injection of the DAD1 antagonist SCH 23390 reduces saccharin seeking in rats. Appetite. 2016 Oct 1 105:8-13.
- VU 0155041 sodium salt
Catalog No.:BCC7642
CAS No.:1259372-69-4
- JP 1302 dihydrochloride
Catalog No.:BCC7449
CAS No.:1259314-65-2
- SAMS Peptide
Catalog No.:BCC5745
CAS No.:125911-68-4
- 24-Methylenecycloartanol acetate
Catalog No.:BCN6137
CAS No.:1259-94-5
- (+)-Glaucarubinone
Catalog No.:BCN7956
CAS No.:1259-86-5
- LY2940680
Catalog No.:BCC3935
CAS No.:1258861-20-9
- 9-O-Ethyldeacetylorientalide
Catalog No.:BCN7311
CAS No.:1258517-60-0
- Deacetylorientalide
Catalog No.:BCN7310
CAS No.:1258517-59-7
- Parathyroid Hormone (1-34), bovine
Catalog No.:BCC1040
CAS No.:12583-68-5
- Pristimerin
Catalog No.:BCN2315
CAS No.:1258-84-0
- CEP-33779
Catalog No.:BCC2199
CAS No.:1257704-57-6
- Amikacin hydrate
Catalog No.:BCC4621
CAS No.:1257517-67-1
- 10-O-Ethylcannabitriol
Catalog No.:BCN7312
CAS No.:1259515-25-7
- Griseofulvin
Catalog No.:BCC5327
CAS No.:126-07-8
- Solasodine
Catalog No.:BCN2346
CAS No.:126-17-0
- Sarsasapogenin
Catalog No.:BCN1269
CAS No.:126-19-2
- Oxethazaine
Catalog No.:BCC3832
CAS No.:126-27-2
- Polygalic acid
Catalog No.:BCN3172
CAS No.:1260-04-4
- Phlegmanol C
Catalog No.:BCN6138
CAS No.:1260-05-5
- Carminic acid
Catalog No.:BCN6541
CAS No.:1260-17-9
- 28-Deoxonimbolide
Catalog No.:BCN4717
CAS No.:126005-94-5
- TAK-438
Catalog No.:BCC1182
CAS No.:1260141-27-2
- 3-Oxo-21alpha-methoxy-24,25,26,27-tetranortirucall-7-ene-23(21)-lactone
Catalog No.:BCN7028
CAS No.:1260173-73-6
- TCS 21311
Catalog No.:BCC2443
CAS No.:1260181-14-3
L-dopa induces opposing effects on pain in intact rats: (-)-sulpiride, SCH 23390 or alpha-methyl-DL-p-tyrosine methylester hydrochloride reveals profound hyperalgesia in large antinociceptive doses.[Pubmed:1432683]
J Pharmacol Exp Ther. 1992 Nov;263(2):470-9.
The present study shows that during the time course of the action of single doses, L-dopa induces multiphasic opposing effects on pain, recorded as vocalization during the presentation of electrical stimulation applied to the tail of normal rats. This indicates that two or more functional systems contribute to produce the net response. A small dose (15 mg/kg) of L-dopa facilitates pain slightly, whereas larger doses (100-200 mg/kg) can produce an antinociceptive effect following an initial hyperalgesia. Moreover, profound hyperalgesia is revealed by either dopamine (DA) D1 and D2 receptor blockade by means of SCH 23390 [R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetra-hydro-1H- 3-benzazepine hydrochloride] or (-)-sulpiride, respectively, as well as after a reduction of the presynaptic synthesis of catecholamines after pretreatment of the animals with the tyrosine hydroxylase inhibitor alpha-methyl-DL-p-tyrosine (alpha-MPT). The enhancement of L-dopa's hyperalgesic effect after SCH 23390 treatment is maximal already at the onset of the effects, whereas (-)-sulpiride or alpha-methyl-DL-p-tyrosine precipitates the hyperalgesia after a certain temporal delay during defined phases of the time course of the effects of large L-dopa doses. The D1 receptor agonist (+)-SKF 38393 potentiates both the hyperalgesic and antinociceptive effects of 100 mg/kg of L-dopa. It is suggested that L-dopa's net effect on pain is modulated from concentration-dependent, opposing effector systems involving both DA stimulatory and inhibitory receptor mechanisms. At high dosing, activation of D2 receptors enhancing DA functional activity produces an antinociceptive response that normally outweighs the hyperalgesia, but this effect becomes dissociable with inhibition of central DA activity.
Classic D1 dopamine receptor antagonist R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) directly inhibits G protein-coupled inwardly rectifying potassium channels.[Pubmed:12065762]
Mol Pharmacol. 2002 Jul;62(1):119-26.
R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) is a widely used, highly selective antagonist of D1 dopamine receptors. While investigating the crosstalk between D1 and D3 dopamine receptor signaling pathways, we discovered that in addition to being a D1 receptor antagonist, SCH23390 and related compounds inhibit G protein-coupled inwardly rectifying potassium (GIRK) channels. We present evidence that SCH23390 blocks endogenous GIRK currents induced by either somatostatin or D3 dopamine receptors in AtT-20 cells (IC50, 268 nM). The inhibition is receptor-independent because constitutive GIRK currents in Chinese hamster ovary cells expressing only GIRK channels are also blocked by SCH23390. The inhibition of GIRK channels is somewhat selective because members of the closely related Kir2.0 family of inwardly rectifying potassium channels, as well as various endogenous cationic currents present in AtT-20 cells, are not affected. In addition, in current clamp recordings, SCH23390 can depolarize the membrane potential and induce AtT-20 cells to fire action potentials, indicating potential physiological significance of the GIRK channel inhibition. To identify the chemical features that contribute to GIRK channel block, we tested several structurally related compounds [SKF38393, R-(+)-7-chloro-8-hydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (nor-methyl-SCH23390), and R-(+)-2,3,4,5-tetrahydro-8-iodo-3-methyl-5-phenyl-1H-3-benzazepin-7-ol hydrochloride (iodo-SCH23390)], and our results indicate that the halide atom is critical for blocking GIRK channels. Taken together, our results suggest that SCH23390 and related compounds might provide the basis for designing novel GIRK channel-selective blockers. Perhaps more importantly, some studies that have exclusively used SCH23390 to probe D1 receptor function or as a diagnostic of D1 receptor involvement may need to be reevaluated in light of these results.
SCH 23390: the first selective dopamine D1-like receptor antagonist.[Pubmed:11830757]
CNS Drug Rev. 2001 Winter;7(4):399-414.
SCH 23390, the halobenzazepine (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5- tetrahydro-1H-3-benzazepine, is a highly potent and selective dopamine D1-like receptor antagonist with a K(i) of 0.2 and 0.3 nM for the D1 and D5 dopamine receptor subtypes, respectively. In vitro, it also binds with high affinity to the 5-HT2 and 5-HT1C serotonin receptor subtypes. However, the doses required to induce a similar response in vivo are greater than 10-fold higher than those required to induce a D1-mediated response. Previous in vivo pharmacological studies with SCH 23390 have shown it to abolish generalized seizures evoked by the chemoconvulsants: pilocarpine and soman. These studies provide evidence of the potential importance of D1-like dopaminergic receptor mechanisms in facilitating the initiation and spread of seizures. The inference from a majority of studies is that the activation of dopamine D1 receptors facilitates seizure activity, whereas activation of D2 receptors may inhibit the development of seizures. SCH 23390 has also been used in studies of other neurological disorders in which the dopamine system has been implicated, such as psychosis and Parkinson's disease. Apart from the study of neurological disorders, SCH 23390 has been extensively used as a tool in the topographical determination of brain D1 receptors in rodents, nonhuman primates, and humans. In summary, SCH 23390 has been a major tool in gaining a better understanding of the role of the dopamine system, more specifically the D1 receptor, in neurological function and dysfunction.
The "selective" dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors.[Pubmed:11465634]
Psychopharmacology (Berl). 2001 Jun;156(1):58-62.
RATIONALE: The benzazepine and "selective" dopamine D1 receptor antagonist, SCH23390 [(R)-(+)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-benzazepine-7-ol], shows significant affinity at native serotonin (5-HT)2C receptors. OBJECTIVES: We examined its functional actions at cloned human (h)5-HT2C receptors (VSV isoform) stably expressed in CHO cells. METHODS: Since 5-HT2C receptors are positively coupled to phospholipase C (PLC), their activation was determined by depletion of membrane-bound pools of pre-labelled [3H]phosphotidylinositol ([3H]PI). RESULTS: SCH23390 showed high affinity (Ki, 9.3 nM) at h5-HT2C sites and depleted [3H]PI with an EC50 of 2.6 nM. Its efficacy was equivalent to that of 5-HT. [3H]PI depletion elicited by SCH23390 was concentration-dependently abolished by the selective 5-HT2C antagonist, SB242,084, with a K(B) of 0.55 nM. Further, in the presence of a fixed concentration of SB242,084 (10 nM), the concentration-response curve for SCH23390 was shifted to the right without loss of maximal effect, yielding a K(B) of 0.57 nM. CONCLUSIONS: SCH23390 is a potent and high efficacy agonist at h5-HT2C receptors. Activation of 5-HT2C receptors by SCH23390 may contribute to its functional properties both in animals and in humans.
Activation of the 5-HT1C receptor expressed in Xenopus oocytes by the benzazepines SCH 23390 and SKF 38393.[Pubmed:1687364]
Br J Pharmacol. 1991 Dec;104(4):1038-44.
1. A cloned 5-HT1C receptor expressed in Xenopus laevis oocytes was used to characterize the action of four dopamine D1-selective benzazepines at the 5-HT1C receptor. Additionally, the apparent binding of the D1-selective benzazepines to 5-HT1C receptors was measured in the choroid plexus of the pig. 2. In voltage-clamped oocytes expressing the cloned 5-HT1C receptor, 5-hydroxytryptamine (5-HT) elicited a characteristic inward current response with an EC50 of 13 nM. SCH 23390 acted as a stereoselective agonist (or partial agonist) with an EC50 of about 550 nM. SKF 38393 (1 microM-1 mM), SKF 77434 (100 microM), and SKF 82958 (100 microM) also acted as agonists (or partial agonists) at the cloned 5-HT1C receptor. SKF 38393 was not stereoselective at the 5-HT1C receptor. 3. The response to SCH 23390 activated slowly and, although the response contained many oscillations characteristic of the activation of the phosphatidylinositol signal transduction system, SCH 23390 rarely elicited the rapid spike-like response seen routinely in response to 5-HT. However, the responses to SKF 38393, SKF 77434, and SKF 82958 were identical in appearance to the response to 5-HT, except that the responses to the benzazepines were smaller. These comparisons were made by applying both a benzazepine and 5-HT to each individual oocyte expressing the cloned 5-HT1C receptor. 4. Consistent with the responses measured in oocytes, SCH 23390 bound stereoselectively to 5-HT1C receptors in the choroid plexus of the pig (Ki = 6.3 nM), and SKF 38393 bound non-stereoselectively with lower affinity (Ki = 2.0-2.2 microM).5. It is concluded that while these benzazepines demonstrate selectivity for the dopamine D1 receptor, they also can act as agonists or partial agonists at the 5-HT1c receptor in situ and as expressed in Xenopus oocytes. The oocyte expression system is useful for studies of the functional pharmacology of these 5-HTic receptors. Information about the pharmacological actions and variations in stereoselectivity among dopamine and 5-HT receptors should be of interest in modelling the interactions of ligands with these G-protein coupled receptors, and in the testing of such models through receptor mutagenesis.


