SB-3CTgelatinases inhibitor, potent and selective CAS# 292605-14-2 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 292605-14-2 | SDF | Download SDF |
| PubChem ID | 9883002 | Appearance | Powder |
| Formula | C15H14O3S2 | M.Wt | 306.40 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (163.19 mM) *"≥" means soluble, but saturation unknown. | ||
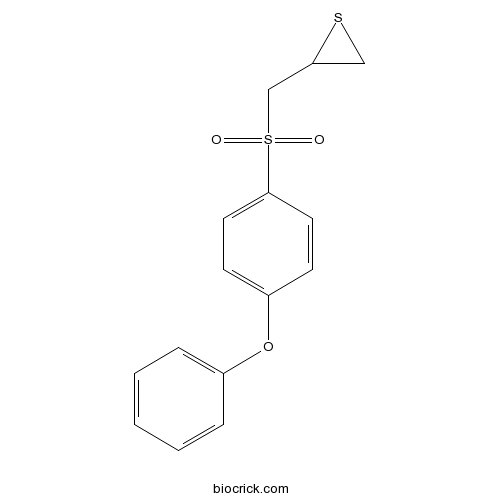
| Chemical Name | 2-[(4-phenoxyphenyl)sulfonylmethyl]thiirane | ||
| SMILES | C1C(S1)CS(=O)(=O)C2=CC=C(C=C2)OC3=CC=CC=C3 | ||
| Standard InChIKey | LSONWRHLFZYHIN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H14O3S2/c16-20(17,11-14-10-19-14)15-8-6-13(7-9-15)18-12-4-2-1-3-5-12/h1-9,14H,10-11H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective high affinity MMP2 inhibitor (Ki = 28 nM); also inhibits MMP9 (Ki = 400 nM). Active in vivo, neuroprotective and blood-brain barrier permeable. |

SB-3CT Dilution Calculator

SB-3CT Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2637 mL | 16.3185 mL | 32.6371 mL | 65.2742 mL | 81.5927 mL |
| 5 mM | 0.6527 mL | 3.2637 mL | 6.5274 mL | 13.0548 mL | 16.3185 mL |
| 10 mM | 0.3264 mL | 1.6319 mL | 3.2637 mL | 6.5274 mL | 8.1593 mL |
| 50 mM | 0.0653 mL | 0.3264 mL | 0.6527 mL | 1.3055 mL | 1.6319 mL |
| 100 mM | 0.0326 mL | 0.1632 mL | 0.3264 mL | 0.6527 mL | 0.8159 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SB-3CT is a potent and selective inhibitor of gelatinases with Ki values of 13.9 and 600 nM for MMP-2 and MMP-9, respectively.
Gelatinases A (MMP-2) and B (MMP-9) belong to matrix metalloproteinases family and are involved in tumor metastasis and angiogenesis by hydrolyzing extracellular matrix [1].
SB-3CT is a potent and selective gelatinases inhibitor. SB-3CT was a novel mechanism-based inhibitor that directly bound to the catalytic zinc ion of MMP-2 [1]. SB-3CT inhibited purified mouse MMP-9 with Ki value of 120 nM [2].
In mice bearing T-cell lymphoma L-CI.5s cells, SB-3CT reduced the number of liver metastases in a dose dependent way and reduced the number of tumor colonies by >70% at 50 mg/kg/d. SB-3CT (50 mg/kg/d) also significantly reduced colony size and the number of PCNA positive tumor cells in the livers. These results suggested that SB-3CT effectively inhibited tumor cell extravasation and exhibited an antiproliferative effect. SB-3CT completely inhibited MMP-mediated gelatinolytic activity through the inhibition of both MMP-2 and MMP-9. Also, SB-3CT increased survival [2]. In a transient focal cerebral ischemia mice model, SB-3CT inhibited laminin cleavage mediated by MMP-9 and then rescued neurons from apoptosis [3].
References:
[1]. Kleifeld O, Kotra LP, Gervasi DC, et al. X-ray absorption studies of human matrix metalloproteinase-2 (MMP-2) bound to a highly selective mechanism-based inhibitor. comparison with the latent and active forms of the enzyme. J Biol Chem, 2001, 276(20): 17125-17131.
[2]. Krüger A, Arlt MJ, Gerg M, et al. Antimetastatic activity of a novel mechanism-based gelatinase inhibitor. Cancer Res, 2005, 65(9): 3523-3526.
[3]. Gu Z, Cui J, Brown S, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci, 2005, 25(27): 6401-6408.
- Dehydrotrametenolic acid
Catalog No.:BCN2718
CAS No.:29220-16-4
- L-Kynurenine
Catalog No.:BCC3899
CAS No.:2922-83-0
- H-DL-Phe(4-NO2)-OH
Catalog No.:BCC3279
CAS No.:2922-40-9
- Adenine HCl
Catalog No.:BCC4453
CAS No.:2922-28-3
- Methylprednisolone hemisuccinate
Catalog No.:BCC9044
CAS No.:2921-57-5
- 3',6'-Bis(diethylamino)-2-(4-nitrophenyl)spiro[isoindole-1,9'-xanthene]-3-one
Catalog No.:BCC8597
CAS No.:29199-09-5
- Shegansu B
Catalog No.:BCN3381
CAS No.:291535-65-4
- [Ala11,22,28]VIP
Catalog No.:BCC5754
CAS No.:291524-04-4
- 1-Methoxyberberine
Catalog No.:BCN7373
CAS No.:29133-52-6
- (+)-Affinisine
Catalog No.:BCN3520
CAS No.:2912-11-0
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- 25,26-Dihydroxyvitamin D3
Catalog No.:BCC4201
CAS No.:29261-12-9
- L-685,458
Catalog No.:BCC2344
CAS No.:292632-98-5
- Deoxyelephantopin
Catalog No.:BCN4655
CAS No.:29307-03-7
- Genipin-1-O-gentiobioside
Catalog No.:BCN5349
CAS No.:29307-60-6
- Licarbazepine
Catalog No.:BCC7794
CAS No.:29331-92-8
- Tetrahydropalmatine
Catalog No.:BCN6310
CAS No.:2934-97-6
- Ciclopirox
Catalog No.:BCC4899
CAS No.:29342-05-0
- Olean-12-ene-3,11-dione
Catalog No.:BCN5195
CAS No.:2935-32-2
- H-Phg-OH
Catalog No.:BCC3310
CAS No.:2935-35-5
- SR3335
Catalog No.:BCC1964
CAS No.:293753-05-6
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- Thevetiaflavone
Catalog No.:BCN4024
CAS No.:29376-68-9
MMP-9 inhibitor SB-3CT attenuates behavioral impairments and hippocampal loss after traumatic brain injury in rat.[Pubmed:24661104]
J Neurotrauma. 2014 Jul 1;31(13):1225-34.
The aim of this study was to evaluate the potential efficacy of SB-3CT, a matrix metallopeptidase 9 inhibitor, on behavioral and histological outcomes after traumatic brain injury (TBI) in rats. Adult male Sprague-Dawley rats were randomly divided into three groups (n=15/group): TBI with SB-3CT treatment, TBI with saline, and sham injury. The TBI model was induced by a fluid percussion TBI device. SB-3CT (50 mg/kg in 10% dimethyl sulfoxide) was administered intraperitoneally at 30 min, 6 h, and 12 h after the TBI. Motor function (beam-balance/beam-walk tests) and spatial learning/memory (Morris water maze) were assessed on post-operative Days 1-5 and 11-15, respectively. Fluoro-Jade staining, immunofluorescence, and cresyl violet-staining were carried out for histopathological evaluation at 24 h, 72 h, and 15 days after TBI, respectively. It was shown that TBI can result in significant behavioral deficit induced by acute neurodegeneration, increased expression of cleaved caspase-3, and long-term neuronal loss. SB-3CT intervention via the current regime provides robust behavioral protection and hippocampal neurons preservation from the deleterious effects of TBI. Hence, the efficacy of SB-3CT on TBI prognosis could be ascertained. It is believed that the current study adds to the growing literature in identifying SB-3CT as a potential therapy for human brain injury.
QM/MM Studies of the Matrix Metalloproteinase 2 (MMP2) Inhibition Mechanism of (S)-SB-3CT and its Oxirane Analogue.[Pubmed:21076643]
J Chem Theory Comput. 2010 Nov 9;6(11):3580-3587.
SB-3CT, (4-phenoxyphenylsulfonyl)methylthiirane, is a potent, mechanism-based inhibitor of the gelatinase sub-class of the matrix metalloproteinase (MMP) family of zinc proteases. The gelatinase MMPs are unusual in that there are several examples where both enantiomers of a racemic inhibitor have comparable inhibitory abilities. SB-3CT is one such example. Here, the inhibition mechanism of the MMP2 gelatinase by the (S)-SB-3CT enantiomer and its oxirane analogue is examined computationally, and compared to the mechanism of (R)-SB-3CT. Inhibition of MMP2 by (R)-SB-3CT was shown previously to involve enzyme-catalyzed C-H deprotonation adjacent to the sulfone, with concomitant opening by beta-elimination of the sulfur of the three-membered thiirane ring. Similarly to the R enantiomer, (S)-SB-3CT was docked into the active site of MMP2, followed by molecular dynamics simulation to prepare the complex for combined quantum mechanics and molecular mechanics (QM/MM) calculations. QM/MM calculations with B3LYP/6-311+G(d,p) for the QM part (46 atoms) and the AMBER force field for the MM part were used to compare the reaction of (S)-SB-3CT and its oxirane analogue in the active site of MMP2 (9208 atoms). These calculations show that the barrier for the proton abstraction coupled ring opening reaction of (S)-SB-3CT in the MMP2 active site is 4.4 kcal/mol lower than its oxirane analogue, and the ring opening reaction energy of (S)-SB-3CT is only 1.6 kcal/mol less exothermic than its oxirane analogue. Calculations also show that the protonation of the ring-opened products by water is thermodynamically much more favorable for the alkoxide obtained from the oxirane, than for the thiolate obtained from the thiirane. In contrast to (R)-SB-3CT and the R-oxirane analogue, the double bonds of the ring-opened products of (S)-SB-3CT and its S-oxirane analogue have the cis-configuration. Vibrational frequency and intrinsic reaction path calculations on a reduced size QM/MM model (2747 atoms) provide additional insight into the mechanism. These calculations yield 5.9 and 6.7 for the deuterium kinetic isotope effect for C-H bond cleavage in the transition state for the R and S enantiomers of SB-3CT, in good agreement with the experimental results.
DFT studies of the ring-opening mechanism of SB-3CT, a potent inhibitor of matrix metalloproteinase 2.[Pubmed:19445474]
Org Lett. 2009 Jun 18;11(12):2559-62.
SB-3CT is a 2-[(arylsulfonyl)methyl]thiirane that achieves potent inhibition, by a thiirane-opening mechanism, of the MMP2 and MMP9 zinc metalloproteases. The deprotonation mechanism for thiirane opening of SB-3CT and for the opening of its oxirane analogue, both relevant to the inhibition of MMP2, was investigated computationally using the acetate anion as the Bronsted base and in methanol and acetonitrile as solvents. The activation barriers for the reaction show a significant stereoelectronic effect. The lowest energy paths have the breaking C-H bond gauche to both sulfone oxygens and with this C-H bond anti to the breaking C-S bond of the thiirane. The calculated primary isotope effect agrees with experimental data.
Matrix metalloproteinase 2 (MMP2) inhibition: DFT and QM/MM studies of the deprotonation-initialized ring-opening reaction of the sulfoxide analogue of SB-3CT.[Pubmed:20039633]
J Phys Chem B. 2010 Jan 21;114(2):1030-7.
(4-Phenoxyphenylsulfonyl)methylthiirane (SB-3CT) is the selective inhibitor of matrix metalloproteinase 2 (MMP2). The inhibition mechanism of MMP2 by SB-3CT involves C-H deprotonation with concomitant opening of the three-membered heterocycle. In this study, the energetics of the deprotonation-induced ring-opening of (4-phenoxyphenylsulfinyl)methylthiirane, the sulfoxide analogue of SB-3CT, are examined computationally using DFT and QM/MM calculations. A model system, 2-(methylsulfinylmethyl)thiirane, is used to study the stereoelectronic and conformational effects of reaction barriers in methanol. For the model system in methanol solution (using the polarizable continuum model), the reaction barriers range from 17 to 23 kcal/mol with significant stereoelectronic effects. However, the lowest barriers of the (R,R) and (S,R) diastereomers are similar. Two diastereomers of the sulfoxide analogue of SB-3CT are studied in the active site of MMP2 by QM/MM methods with an accurate partial charge fitting procedure. The ring-opening reactions of these two diastereomers have similar reaction energetics. Both are exothermic from the reactant to the ring-opening product (thiolate). The protonation of the thiolate by a water molecule is endothermic in both cases. However, the deprotonation/ring-opening barriers in the MMP2 active site using QM/MM methods for the (R,R) and (S,R) inhibitions are quite different (23.3 and 28.5 kcal/mol, respectively). The TSs identified in QM/MM calculations were confirmed by vibrational frequency analysis and following the reaction path. The (R,R) diastereomer has a hydrogen bond between the sulfoxide oxygen and the backbone NH of Leu191, while the (S,R) has a hydrogen bond between the sulfoxide oxygen and a water molecule. The dissimilar strengths of these hydrogen bonds as well as minor differences in the TS structures contribute to the difference between the barriers. Compared to SB-3CT, both diastereomers of the sulfoxide analogue have higher reaction barriers and have less exothermic reaction energies. This agrees well with the experiments, where SB-3CT is a more effective inhibitor of MMP2 than its sulfoxide analogue.


