Pteroside ACAS# 35910-15-7 |
Quality Control & MSDS
Number of papers citing our products

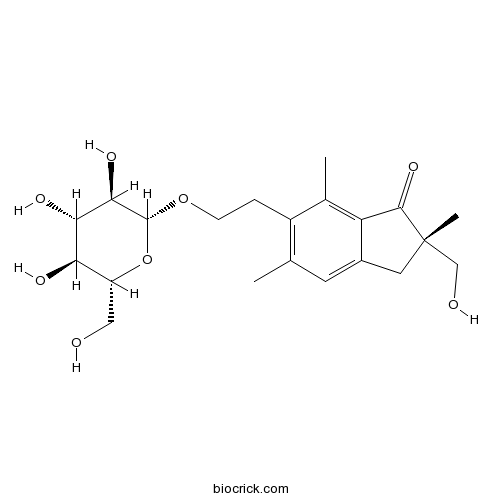
Chemical structure

3D structure
| Cas No. | 35910-15-7 | SDF | Download SDF |
| PubChem ID | 169727 | Appearance | Powder |
| Formula | C21H30O8 | M.Wt | 410.5 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-(hydroxymethyl)-2,5,7-trimethyl-6-[2-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyethyl]-3H-inden-1-one | ||
| SMILES | CC1=C(C(=C2C(=C1)CC(C2=O)(C)CO)C)CCOC3C(C(C(C(O3)CO)O)O)O | ||
| Standard InChIKey | UTBLUTBCAVVCIO-HPCBLLCTSA-N | ||
| Standard InChI | InChI=1S/C21H30O8/c1-10-6-12-7-21(3,9-23)19(27)15(12)11(2)13(10)4-5-28-20-18(26)17(25)16(24)14(8-22)29-20/h6,14,16-18,20,22-26H,4-5,7-9H2,1-3H3/t14-,16-,17+,18-,20-,21+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Chemical and biologically active constituents of Pteris multifida.[Pubmed: 18553125]J Nat Med. 2008 Oct;62(4):452-5.A new compound, 4-caffeoyl quinic acid 5-O-methyl ether (2), together with 12 known compounds--identified as (2R,3R)-pterosin L 3-O-beta-D-glucopyrannoside (3), beta-sitosterol beta-D-glucopyranoside (4), apigenin 7-O-beta-D-glucopyranoside (5), luteolin 7-O-beta-D-glucopyranoside (6), sucrose (7), caffeic acid (8), pterosin C 3-O-beta-D-glucopyranoside (9), pteroside C (10), 4,5-dicaffeoyl quinic acid (11), Pteroside A (12), wallichoside (13) and (2S)-5,7,3',5'-tetrahydroxyflavanone (14)--were isolated from Pteris multifida.
|
| Structure Identification | Phytochemistry. 2016 Aug;128:82-94.Isolation and characterisation of 13 pterosins and pterosides from bracken (Pteridium aquilinum (L.) Kuhn) rhizome.[Pubmed: 27177933 ]Systematic phytochemical investigations of the underground rhizome of Pteridium aquilinum (L.) Kuhn (Dennstaedtiaceae) afforded thirty-five pterosins and pterosides. |

Pteroside A Dilution Calculator

Pteroside A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4361 mL | 12.1803 mL | 24.3605 mL | 48.7211 mL | 60.9013 mL |
| 5 mM | 0.4872 mL | 2.4361 mL | 4.8721 mL | 9.7442 mL | 12.1803 mL |
| 10 mM | 0.2436 mL | 1.218 mL | 2.4361 mL | 4.8721 mL | 6.0901 mL |
| 50 mM | 0.0487 mL | 0.2436 mL | 0.4872 mL | 0.9744 mL | 1.218 mL |
| 100 mM | 0.0244 mL | 0.1218 mL | 0.2436 mL | 0.4872 mL | 0.609 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3,3-Dimethoxy-5α-androstan-17-one
Catalog No.:BCC8594
CAS No.:3591-19-3
- Onjisaponin B
Catalog No.:BCN2741
CAS No.:35906-36-6
- Boc-His(Tos)-OH
Catalog No.:BCC3402
CAS No.:35899-43-5
- Ligustroside
Catalog No.:BCN5310
CAS No.:35897-92-8
- Boc-Arg-OH.HCl.H2O
Catalog No.:BCC3053
CAS No.:35897-34-8
- Vestitol
Catalog No.:BCN5309
CAS No.:35878-41-2
- Novokinin
Catalog No.:BCC6051
CAS No.:358738-77-9
- Apocynol A
Catalog No.:BCN4642
CAS No.:358721-33-2
- H-Trp-OBzl.HCl
Catalog No.:BCC3113
CAS No.:35858-81-2
- Etazolate hydrochloride
Catalog No.:BCC6648
CAS No.:35838-58-5
- 3-Epicabraleahydroxylactone
Catalog No.:BCN5308
CAS No.:35833-72-8
- Cabraleahydroxylactone acetate
Catalog No.:BCN5307
CAS No.:35833-70-6
- Pterosin A
Catalog No.:BCN8147
CAS No.:35910-16-8
- NECA
Catalog No.:BCC5804
CAS No.:35920-39-9
- Vicenin -1
Catalog No.:BCN3012
CAS No.:35927-38-9
- Pterosin C
Catalog No.:BCN7104
CAS No.:35938-43-3
- Triciribine
Catalog No.:BCC3872
CAS No.:35943-35-2
- Pteroside D
Catalog No.:BCN5311
CAS No.:35943-38-5
- Anchustrigosine
Catalog No.:BCC8185
CAS No.:
- 3-Hydroxy-11-ursen-28,13-olide
Catalog No.:BCN5312
CAS No.:35959-05-8
- 3-Acetoxy-11-ursen-28,13-olide
Catalog No.:BCN5313
CAS No.:35959-08-1
- Inotodiol
Catalog No.:BCN3331
CAS No.:35963-37-2
- Triptotin F
Catalog No.:BCN3482
CAS No.:359630-36-7
- Pterosin G
Catalog No.:BCN8148
CAS No.:35964-50-2
Isolation and characterisation of 13 pterosins and pterosides from bracken (Pteridium aquilinum (L.) Kuhn) rhizome.[Pubmed:27177933]
Phytochemistry. 2016 Aug;128:82-94.
Systematic phytochemical investigations of the underground rhizome of Pteridium aquilinum (L.) Kuhn (Dennstaedtiaceae) afforded thirty-five pterosins and pterosides. By detailed analysis of one- and two-dimensional nuclear magnetic resonance spectroscopy, circular dichroism (CD) and high-resolution mass spectrometric data, thirteen previously undescribed pterosins and pterosides have been identified. Interestingly, for the first time 12-O-beta-D-glucopyranoside substituted pterosins, rhedynosides C and D, and the sulfate-containing pterosin, rhedynosin H, alongside the two known compounds, histiopterosin A and (2S)-Pteroside A2, were isolated from the rhizomes of subsp. aquilinum of bracken. In addition, six-membered cyclic ether pterosins and pterosides, rhedynosin A and rhedynoside A, are the first examples of this type of pterosin-sesquiterpenoid. Additionally, the three previously reported compounds (rhedynosin I, (2S)-2-hydroxymethylpterosin E and (2S)-12-hydroxypterosin A) were obtained for the first time from plants as opposed to mammalian metabolic products. Single crystal X-ray diffraction analysis was applied to the previously undescribed compounds (2R)-rhedynoside B, (2R)-pteroside B and (2S)-pteroside K, yielding the first crystal structures for pterosides, and three known pterosins, (2S)-pterosin A, trans-pterosin C and cis-pterosin C. Rhedynosin C is the only example of the cyclic lactone pterosins with a keto group at position C-14. Six selected pterosins ((2S)-pterosin A, (2R)-pterosin B and trans-pterosin C) and associated glycosides ((2S)-Pteroside A, (2R)-pteroside B and pteroside Z) were assessed for their anti-diabetic activity using an intestinal glucose uptake assay; all were found to be inactive at 300 muM.
Chemical and biologically active constituents of Pteris multifida.[Pubmed:18553125]
J Nat Med. 2008 Oct;62(4):452-5.
A new compound, 4-caffeoyl quinic acid 5-O-methyl ether (2), together with 12 known compounds--identified as (2R,3R)-pterosin L 3-O-beta-D-glucopyrannoside (3), beta-sitosterol beta-D-glucopyranoside (4), apigenin 7-O-beta-D-glucopyranoside (5), luteolin 7-O-beta-D-glucopyranoside (6), sucrose (7), caffeic acid (8), pterosin C 3-O-beta-D-glucopyranoside (9), pteroside C (10), 4,5-dicaffeoyl quinic acid (11), Pteroside A (12), wallichoside (13) and (2S)-5,7,3',5'-tetrahydroxyflavanone (14)--were isolated from Pteris multifida. The structure of the new compound was determined by means of physical, chemical and spectroscopic evidence. Compounds 5 and 6 were the main constituents of the plant, with yields of 0.19% and 0.16%, respectively. The cytotoxic activities of 2, 3, and 9-13 were evaluated against a human cell line (KB cells). Among the isolated compounds, pterosin C 3-O-beta-D-glucopyrannoside (9) and 4,5-dicaffeoylquinic acid (11) showed a significant selective cytotoxicity (IC(50) 2.35 and 5.38, respectively), while moderate activity was observed for compound 2 (IC(50) 12.3). The chemosystematics of Pteris species is also discussed.


