PorphyroxineCAS# 18104-24-0 |
Quality Control & MSDS
Number of papers citing our products

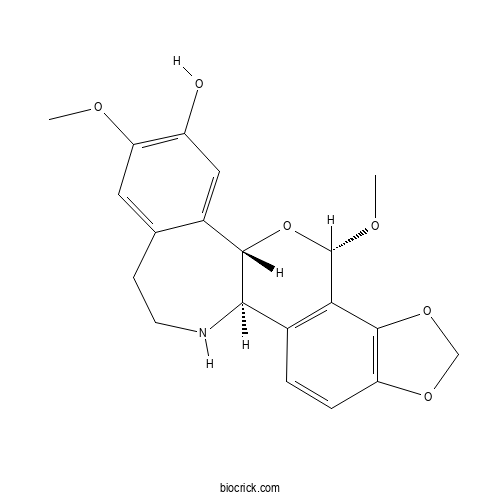
Chemical structure

3D structure
| Cas No. | 18104-24-0 | SDF | Download SDF |
| PubChem ID | 12309641 | Appearance | Powder |
| Formula | C20H21NO6 | M.Wt | 371.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Papaverrubine D | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,11S,13S)-11,17-dimethoxy-6,8,12-trioxa-22-azapentacyclo[11.9.0.02,10.05,9.014,19]docosa-2(10),3,5(9),14,16,18-hexaen-16-ol | ||
| SMILES | COC1C2=C(C=CC3=C2OCO3)C4C(O1)C5=CC(=C(C=C5CCN4)OC)O | ||
| Standard InChIKey | YLUOVOKBMSLYGX-HBFSDRIKSA-N | ||
| Standard InChI | InChI=1S/C20H21NO6/c1-23-15-7-10-5-6-21-17-11-3-4-14-19(26-9-25-14)16(11)20(24-2)27-18(17)12(10)8-13(15)22/h3-4,7-8,17-18,20-22H,5-6,9H2,1-2H3/t17-,18+,20+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Porphyroxine Dilution Calculator

Porphyroxine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6925 mL | 13.4626 mL | 26.9251 mL | 53.8503 mL | 67.3129 mL |
| 5 mM | 0.5385 mL | 2.6925 mL | 5.385 mL | 10.7701 mL | 13.4626 mL |
| 10 mM | 0.2693 mL | 1.3463 mL | 2.6925 mL | 5.385 mL | 6.7313 mL |
| 50 mM | 0.0539 mL | 0.2693 mL | 0.5385 mL | 1.077 mL | 1.3463 mL |
| 100 mM | 0.0269 mL | 0.1346 mL | 0.2693 mL | 0.5385 mL | 0.6731 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dorsmanin I
Catalog No.:BCN0547
CAS No.:329703-46-0
- Trigonothyrin C
Catalog No.:BCN0546
CAS No.:1203471-31-1
- Ananolignan L
Catalog No.:BCN0545
CAS No.:1280213-70-8
- Trigochinin C
Catalog No.:BCN0544
CAS No.:1210299-33-4
- 6-Hydroxy-5,7,3',4',5'-pentamethoxyflavone
Catalog No.:BCN0543
CAS No.:29043-06-9
- Forestine
Catalog No.:BCN0542
CAS No.:91794-14-8
- 5-Hydroxy-1,7-diphenylhept-6-en-3-one
Catalog No.:BCN0541
CAS No.:155239-30-8
- meso-Hannokinol
Catalog No.:BCN0540
CAS No.:79055-11-1
- 6β-Hydroxyandrost-4-ene-3,17-dione
Catalog No.:BCN0539
CAS No.:63-00-3
- Hedyotol C
Catalog No.:BCN0538
CAS No.:97465-79-7
- (4Z,6E)-5-Hydroxy-1,7-diphenylhepta-4,6-dien-3-one
Catalog No.:BCN0537
CAS No.:478313-96-1
- (Z)-5-Hydroxy-1,7-diphenylhept-4-en-3-one
Catalog No.:BCN0536
CAS No.:856007-75-5
- Chrysin 7-O-neohesperidoside
Catalog No.:BCN0549
CAS No.:35775-46-3
- Methyl 2-(2,3-dihydroxyphenyl)acetate
Catalog No.:BCN0550
CAS No.:168481-87-6
- 2-Hydroxybenzylcyanide
Catalog No.:BCN0551
CAS No.:14714-50-2
- Squamolone
Catalog No.:BCN0552
CAS No.:40451-67-0
- 2''-O-Rhamnosylswertisin
Catalog No.:BCN0553
CAS No.:83889-78-5
- Glochidiolide
Catalog No.:BCN0554
CAS No.:213528-23-5
- (19S,23E)-5β,19-Epoxy-19-methoxycucurbita-6,23,25-trien-3β-ol
Catalog No.:BCN0555
CAS No.:874287-87-3
- Velutinam
Catalog No.:BCN0556
CAS No.:146428-62-8
- Haplamide
Catalog No.:BCN0557
CAS No.:31991-78-3
- Falconeridine
Catalog No.:BCN0558
CAS No.:121880-22-6
- 14-O-Acetylsachaconitine
Catalog No.:BCN0559
CAS No.:102719-98-2
- Sachaconitine
Catalog No.:BCN0560
CAS No.:1361-02-0
UHPLC-MS/MS quantitation of porphyroxine in opium and application of porphyroxine-acetylated products as signature markers for heroin.[Pubmed:30835929]
Drug Test Anal. 2019 Jul;11(7):999-1008.
Porphyroxine, a trace alkaloid in opium, was identified in the early 1800s and isolated/characterized in the 1960s. Recently, two significant Porphyroxine-related byproducts found in the acidic and neutral extracts of illicit heroin were characterized by this laboratory as the N-acetyl-O(14) -desmethyl-epi-Porphyroxine (B) and N,O(8) -diacetyl-O(14) -desmethyl-epi-Porphyroxine (C). The prevalence of the B and C compounds has been consistent in the following order of abundance for the thousands of authentic heroin samples analyzed: Southwest Asia (SWA) > South America (SA) > Southeast Asia (SEA) > Mexico (MEX). In this research, a rapid and efficient ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method was developed to determine the content of Porphyroxine and five primary alkaloids (morphine, codeine, thebaine, noscapine, and papaverine) in opium after extraction with methanol/water (50/50). The method was validated in terms of linearity, accuracy, recovery, and precision for Porphyroxine. The limit of quantitation (LOQ) for Porphyroxine was 2.5 ng/mL. The developed method was successfully applied to a total of 114 authentic opium samples from the major poppy-growing regions. The amount of Porphyroxine was determined at the level of part per thousand ( per thousand) and the relative concentrations to morphine were in the range of 1x10(-4) and 1x10(-2) with an order of SWA > SEA, SA > MEX for its average abundance, which is consistent with the order of the average abundance of its acetylated products (B, C) in illicit heroin. This study reveals the significance of Porphyroxine and its acylated compounds in classifying heroin and opium samples to major geographical regions of production.
Characterization and origin of the 'B' and 'C' compounds in the acid/neutral forensic signatures of heroin - products from the acylation of porphyroxine and subsequent hydrolysis.[Pubmed:26593749]
Drug Test Anal. 2017 Mar;9(3):462-469.
Two significant compounds often found in the gas chromatographic analysis of the acid/neutral extracts from illicit heroin have remained uncharacterized for 30 years. The unknown compounds are referred to as the 'B' and 'C' compounds. It has been postulated that these compounds arise from acetylation of Porphyroxine, a rhoeadine alkaloid found at trace levels in the opium poppy, Papaver somniferum. Porphyroxine was isolated from opium and acetylated to produce N,O(8) -diacetylPorphyroxine. Mild hydrolysis produced N,O(8) -diacetyl-O(14) -desmethyl-epi-Porphyroxine (the C compound) and N-acetyl-O(14) -desmethyl-epi-Porphyroxine (the B compound). Both N,O(8) -diacetyl-O(14) -desmethyl-epi-Porphyroxine and N-acetyl-O(14) -desmethyl-epi-Porphyroxine were determined to be C-14 epimers of Porphyroxine and N,O(8) -diacetylPorphyroxine. The non-epimerized isomers of the B and C compounds were also detected in illicit heroin, but at much lower levels. Chromatographic and spectroscopic data are presented for the aforementioned compounds. The presence/absence and relative concentrations of these compounds is presented for the four types of heroin (Southwest Asian, South American, Southeast Asian, and Mexican). The prevalence of detection for the B and C compounds are Southwest Asian = 92-93%, South American = 64-72%, Southeast Asian = 45-49%, and Mexican South American > Southeast Asian, each by an order of magnitude. These compounds were rarely detected in Mexican heroin. The presence/absence and relative concentrations of these compounds provide pertinent forensic signature characteristics that significantly enhance the final regional classifications. Copyright (c) 2015 John Wiley & Sons, Ltd.


