Pam2CSK4TLR2/6 agonist; induces TNF-α production CAS# 868247-72-7 |
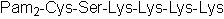
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- AKT inhibitor VIII
Catalog No.:BCC1334
CAS No.:612847-09-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure
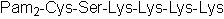
3D structure
| Cas No. | 868247-72-7 | SDF | Download SDF |
| PubChem ID | 71457280 | Appearance | Powder |
| Formula | C65H126N10O12S | M.Wt | 1271.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in 0.25% acetic acid | ||
| Sequence | CSKKKK (Modifications: Cys = S-[2,3-Bis(palmitoyloxy)-(2-R) | ||
| Chemical Name | (2S)-6-amino-2-[[(2S)-6-amino-2-[[(2S)-6-amino-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2R)-2-amino-3-[(2R)-2,3-di(hexadecanoyloxy)propyl]sulfanylpropanoyl]amino]-3-hydroxypropanoyl]amino]hexanoyl]amino]hexanoyl]amino]hexanoyl]amino]hexanoic acid | ||
| SMILES | CCCCCCCCCCCCCCCC(=O)OCC(CSCC(C(=O)NC(CO)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)O)N)OC(=O)CCCCCCCCCCCCCCC | ||
| Standard InChIKey | LJUIOEFZFQRWJG-GHYFRYPYSA-N | ||
| Standard InChI | InChI=1S/C65H126N10O12S/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-41-58(77)86-48-51(87-59(78)42-28-26-24-22-20-18-16-14-12-10-8-6-4-2)49-88-50-52(70)60(79)75-57(47-76)64(83)73-54(38-30-34-44-67)62(81)71-53(37-29-33-43-66)61(80)72-55(39-31-35-45-68)63(82)74-56(65(84)85)40-32-36-46-69/h51-57,76H,3-50,66-70H2,1-2H3,(H,71,81)(H,72,80)(H,73,83)(H,74,82)(H,75,79)(H,84,85)/t51-,52+,53+,54+,55+,56+,57+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Toll-like receptor 2/6 (TLR2/6) agonist. Induces TNF-α production in human mononuclear cells. Also induces proliferation and activation of mouse splenic B cells. |

Pam2CSK4 Dilution Calculator

Pam2CSK4 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SID 7969543
Catalog No.:BCC6026
CAS No.:868224-64-0
- Carasiphenol C
Catalog No.:BCN8251
CAS No.:868168-04-1
- H-Cys-OEt.HCl
Catalog No.:BCC2904
CAS No.:868-59-7
- CRF (human, rat)
Catalog No.:BCC5710
CAS No.:86784-80-7
- Astrasieversianin VII
Catalog No.:BCN2788
CAS No.:86764-11-6
- TG 100572
Catalog No.:BCC1994
CAS No.:867334-05-2
- TG 100801
Catalog No.:BCC1996
CAS No.:867331-82-6
- TG 100572 Hydrochloride
Catalog No.:BCC1995
CAS No.:867331-64-4
- TRC 051384
Catalog No.:BCC7968
CAS No.:867164-40-7
- Linsitinib
Catalog No.:BCC3697
CAS No.:867160-71-2
- Colivelin
Catalog No.:BCC7821
CAS No.:867021-83-8
- Tropanyl 3-hydroxy-4-methoxycinnamate
Catalog No.:BCN1325
CAS No.:86702-58-1
- LY 2365109 hydrochloride
Catalog No.:BCC7677
CAS No.:868265-28-5
- Org 27569
Catalog No.:BCC4411
CAS No.:868273-06-7
- Rhodiolin
Catalog No.:BCC8356
CAS No.:86831-53-0
- Rhodiosin; Herbacetin-7-O-glucorhamnoside
Catalog No.:BCN8478
CAS No.:86831-54-1
- WAY 213613
Catalog No.:BCC7442
CAS No.:868359-05-1
- 2,2,5,5-Tetramethylcyclohexane-1,4-dione
Catalog No.:BCN1324
CAS No.:86838-54-2
- Protosappanin A dimethyl acetal
Catalog No.:BCN6517
CAS No.:868405-37-2
- (+)-Puerol B 2''-O-glucoside
Catalog No.:BCN4561
CAS No.:868409-19-2
- Iristectorigenin B
Catalog No.:BCN8391
CAS No.:39012-01-6
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
- 3',5'-Dimethoxybiphenyl-3-ol
Catalog No.:BCN7529
CAS No.:868666-20-0
- (R)-(-)-α-Methylhistamine dihydrobromide
Catalog No.:BCC5665
CAS No.:868698-49-1
The TLR2/6 ligand PAM2CSK4 is a Th2 polarizing adjuvant in Leishmania major and Brugia malayi murine vaccine models.[Pubmed:26897363]
Parasit Vectors. 2016 Feb 20;9:96.
BACKGROUND: Toll-like receptors (TLRs) play an important role in the innate and adaptive immune responses to pathogens, and are the target of new vaccine adjuvants. TLR2 plays a role in parasite recognition and activation of immune responses during cutaneous leishmaniasis infection, suggesting that TLR2 could be targeted by adjuvants for use in Leishmania vaccines. We therefore explored using Pam2CSK4 (Pam2) and Pam3CSK4 (Pam3) lipopeptide adjuvants, which activate TLR2/6 and TLR2/1 heterodimers respectively, in vaccine models for parasitic infections. METHODS: The use of lipopeptide adjuvants was explored using two vaccine models. For cutaneous leishmaniasis, the lipopeptide adjuvants Pam2 and Pam3 were compared to that of the Th1-driving double-stranded DNA TLR9 agonist CpG for their ability to improve the efficacy of the autoclaved Leishmania major (ALM) vaccine to protect against L. major infection. The ability of Pam2 to enhance the efficacy of a soluble Brugia malayi microfilariae extract (BmMfE) vaccine to protect against filarial infection was also assessed in a peritoneal infection model of B. malayi filariasis. Parasite antigen-specific cellular and humoral immune responses were assessed post-challenge. RESULTS: The use of lipopeptides in ALM-containing vaccines did not provide any protection upon infection with L. major, and Pam2 exacerbated the disease severity in vaccinated mice post-challenge. Pam2, and to a lesser extent Pam3, were able to elevate antigen-specific immune responses post-challenge in this model, but these responses displayed a skewed Th2 phenotype as characterised by elevated levels of IgG1. In the B. malayi vaccine model, the use of Pam2 as an adjuvant with BmMfE induced significant protective immunity to the same level as inclusion of an Alum adjuvant. Here, both Pam2 and Alum were found to enhance antigen-specific antibody production post-challenge, and Pam2 significantly elevated levels of antigen-specific IL-4, IL-5 and IL-13 produced by splenocytes. CONCLUSIONS: These data indicate that TLR2/6-targeting ligands could be considered as adjuvants for vaccines that require robust Th2 and/or antibody-dependent immunity.
Lipoteichoic acid from Lactobacillus plantarum inhibits Pam2CSK4-induced IL-8 production in human intestinal epithelial cells.[Pubmed:25481370]
Mol Immunol. 2015 Mar;64(1):183-9.
Lactobacilli are probiotic bacteria that are considered to be beneficial in the gastrointestinal tract of humans. Although lactobacilli are well known to alleviate intestinal inflammation, the molecular basis of this phenomenon is poorly understood. In this study, we investigated the effect of Lactobacillus plantarum lipoteichoic acid (Lp.LTA), which is a major cell wall component of this species, on the production of interleukin (IL)-8 in human intestinal epithelial Caco-2 cells. Treatment with Pam2CSK4, a synthetic lipopeptide that is known to mimic Gram-positive bacterial lipoproteins as an important virulence factor, significantly induced IL-8 expression in Caco-2 cells. However, neither heat-inactivated L. plantarum nor L. plantarum peptidoglycan inhibited Pam2CSK4-induced IL-8 mRNA expression. In addition, both a deacylated form and a dealanylated form of Lp.LTA failed to inhibit Pam2CSK4-induced IL-8 expression, indicating that the lipid and D-alanine moieties are critical for Lp.LTA-mediated inhibition. Moreover, Lp.LTA inhibited Pam2CSK4-induced activation of p38 kinase, JNK, and NF-kappaB transcription factor by suppressing toll-like receptor 2 activation. Collectively, these results suggest that Lp.LTA exerts anti-inflammatory effects on human intestinal epithelial cells by blocking IL-8 production.
Toll-like receptor agonists synergize with CD40L to induce either proliferation or plasma cell differentiation of mouse B cells.[Pubmed:21991317]
PLoS One. 2011;6(10):e25542.
In a classical dogma, pathogens are sensed (via recognition of Pathogen Associated Molecular Patterns (PAMPs)) by innate immune cells that in turn activate adaptive immune cells. However, recent data showed that TLRs (Toll Like Receptors), the most characterized class of Pattern Recognition Receptors, are also expressed by adaptive immune B cells. B cells play an important role in protective immunity essentially by differentiating into antibody-secreting cells (ASC). This differentiation requires at least two signals: the recognition of an antigen by the B cell specific receptor (BCR) and a T cell co-stimulatory signal provided mainly by CD154/CD40L acting on CD40. In order to better understand interactions of innate and adaptive B cell stimulatory signals, we evaluated the outcome of combinations of TLRs, BCR and/or CD40 stimulation. For this purpose, mouse spleen B cells were activated with synthetic TLR agonists, recombinant mouse CD40L and agonist anti-BCR antibodies. As expected, TLR agonists induced mouse B cell proliferation and activation or differentiation into ASC. Interestingly, addition of CD40 signal to TLR agonists stimulated either B cell proliferation and activation (TLR3, TLR4, and TLR9) or differentiation into ASC (TLR1/2, TLR2/6, TLR4 and TLR7). Addition of a BCR signal to CD40L and either TLR3 or TLR9 agonists did not induce differentiation into ASC, which could be interpreted as an entrance into the memory pathway. In conclusion, our results suggest that PAMPs synergize with signals from adaptive immunity to regulate B lymphocyte fate during humoral immune response.
Physicochemical and biological analysis of synthetic bacterial lipopeptides: validity of the concept of endotoxic conformation.[Pubmed:17308304]
J Biol Chem. 2007 Apr 13;282(15):11030-7.
The importance of the biological function and activity of lipoproteins from the outer or cytoplasmic membranes of Gram-positive and Gram-negative bacteria is being increasingly recognized. It is well established that they are like the endotoxins (lipopolysaccharide (LPS)), which are the main amphiphilic components of the outer membrane of Gram-negative bacteria, potent stimulants of the human innate immune system, and elicit a variety of proinflammatory immune responses. Investigations of synthetic lipopeptides corresponding to N-terminal partial structures of bacterial lipoproteins defined the chemical prerequisites for their biological activity and in particular the number and length of acyl chains and sequence of the peptide part. Here we present experimental data on the biophysical mechanisms underlying lipopeptide bioactivity. Investigation of selected synthetic diacylated and triacylated lipopeptides revealed that the geometry of these molecules (i.e. the molecular conformations and supramolecular aggregate structures) and the preference for membrane intercalation provide an explanation for the biological activities of the different lipopeptides. This refers in particular to the agonistic or antagonistic activity (i.e. their ability to induce cytokines in mononuclear cells or to block this activity, respectively). Biological activity of lipopeptides was hardly affected by the LPS-neutralizing antibiotic polymyxin B, and the biophysical interaction characteristics were found to be in sharp contrast to that of LPS with polymyxin B. The analytical data show that our concept of "endotoxic conformation," originally developed for LPS, can be applied also to the investigated lipopeptide and suggest that the molecular mechanisms of cell activation by amphiphilic molecules are governed by a general principle.
Domain exchange between human toll-like receptors 1 and 6 reveals a region required for lipopeptide discrimination.[Pubmed:16129684]
J Biol Chem. 2005 Nov 4;280(44):36616-25.
Among the 10 human Toll-like receptors (TLRs), TLR2 appears to be unique in its requirement for cooperation with other TLRs, namely TLR1 and TLR6, to mediate cell signaling. Through reconstitution experiments, we have defined more precisely the function of these human TLRs. Human colonic epithelial cells cotransfected with TLR1 and -2 preferentially respond to a synthetic tripalmitoylated bacterial lipopeptide analogue (Pam(3)CSK(4)). However, examination of a wide variety of lipopeptide derivatives indicates that recognition by human TLR1 and -2 does not strictly correlate with the number or position of the acyl chains on the modified cysteine residue. Conversely, human TLR2 and -6 exclusively respond to lipopeptides possessing a diacylglycerol group. Most surprisingly, we have found that an R stereoisomer of diacylated macrophage-activating lipopeptide 2 (MALP-2) exclusively activates epithelial cells through TLR6 and -2 but not through TLR1 and -2. These results suggest that the chirality of the central carbon of the diacylglycerol group of these agonists is a structural determinant for human TLR recognition. Examination of chimeric receptors, generated by domain exchange between TLR1 and -6, has revealed that leucine-rich repeats 9-12 of the extracellular domain enable these receptors to discriminate between structurally similar lipopeptides. However, additional chimeric constructs reveal that this region alone is not sufficient to generate receptors that can functionally cooperate with TLR2. Our results support the idea that TLR1 and TLR6 diverged during evolution to differentially recognize natural lipoprotein structures and that this function has been conserved with respect to the human receptors.


