PadmatinCAS# 80453-44-7 |
Quality Control & MSDS
Number of papers citing our products

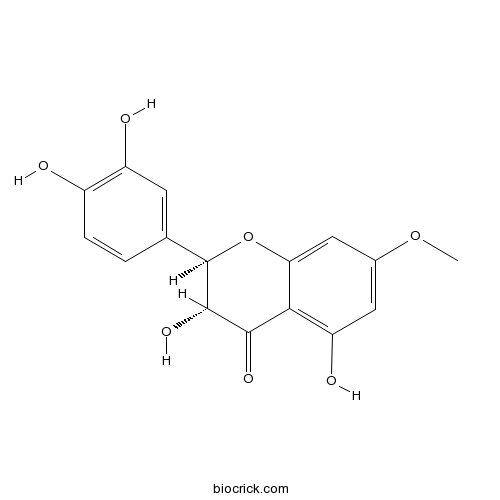
Chemical structure

3D structure
| Cas No. | 80453-44-7 | SDF | Download SDF |
| PubChem ID | 12313901 | Appearance | Powder |
| Formula | C16H14O7 | M.Wt | 318.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R)-2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-7-methoxy-2,3-dihydrochromen-4-one | ||
| SMILES | COC1=CC(=C2C(=C1)OC(C(C2=O)O)C3=CC(=C(C=C3)O)O)O | ||
| Standard InChIKey | MRPJBTFHICBFNE-JKSUJKDBSA-N | ||
| Standard InChI | InChI=1S/C16H14O7/c1-22-8-5-11(19)13-12(6-8)23-16(15(21)14(13)20)7-2-3-9(17)10(18)4-7/h2-6,15-19,21H,1H3/t15-,16+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | Phytochemistry.1992 Jan;31(1):195-197.A eudesmanolide and other constituents fromInula graveolens.[Reference: WebLink]To investigate the chemical constituents of the extract of Galium verum. |

Padmatin Dilution Calculator

Padmatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1417 mL | 15.7085 mL | 31.4169 mL | 62.8338 mL | 78.5423 mL |
| 5 mM | 0.6283 mL | 3.1417 mL | 6.2834 mL | 12.5668 mL | 15.7085 mL |
| 10 mM | 0.3142 mL | 1.5708 mL | 3.1417 mL | 6.2834 mL | 7.8542 mL |
| 50 mM | 0.0628 mL | 0.3142 mL | 0.6283 mL | 1.2567 mL | 1.5708 mL |
| 100 mM | 0.0314 mL | 0.1571 mL | 0.3142 mL | 0.6283 mL | 0.7854 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dynorphin A
Catalog No.:BCC7596
CAS No.:80448-90-4
- gamma-Diasarone
Catalog No.:BCN4339
CAS No.:80434-33-9
- Calcium Levofolinate
Catalog No.:BCC4643
CAS No.:80433-71-2
- Notoginsenoside R2
Catalog No.:BCN3328
CAS No.:80418-25-3
- Notoginsenoside R1
Catalog No.:BCN1097
CAS No.:80418-24-2
- 13-O-p-Coumaroylplumieride
Catalog No.:BCN4338
CAS No.:80416-52-0
- Scorpioidine
Catalog No.:BCN2027
CAS No.:80405-18-1
- 7-Acetylscorpioidine
Catalog No.:BCN2028
CAS No.:80405-17-0
- Protoplumericin A
Catalog No.:BCN4572
CAS No.:80396-57-2
- Z-D-Thr-OH
Catalog No.:BCC2736
CAS No.:80384-27-6
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- 8beta-Tigloyloxyreynosin
Catalog No.:BCN7222
CAS No.:80368-31-6
- Paeoniflorigenone
Catalog No.:BCN3933
CAS No.:80454-42-8
- Sapogenins Glycosides
Catalog No.:BCC5320
CAS No.:8047-15-2
- Fluticasone propionate
Catalog No.:BCC4907
CAS No.:80474-14-2
- Rubifolic acid
Catalog No.:BCN4341
CAS No.:80489-65-2
- Mezlocillin Sodium Monohydrate
Catalog No.:BCC5634
CAS No.:80495-46-1
- Tenacigenin B
Catalog No.:BCN4342
CAS No.:80508-42-5
- Glaucocalyxin B
Catalog No.:BCN8440
CAS No.:80508-81-2
- Veratrine
Catalog No.:BCN8444
CAS No.:8051-02-3
- Euchrestaflavanone A
Catalog No.:BCN3576
CAS No.:80510-05-0
- Grossamide
Catalog No.:BCN4571
CAS No.:80510-06-1
- Cis-N-Feruloyltyramine
Catalog No.:BCN3729
CAS No.:80510-09-4
- 7beta-(3-Ethyl-cis-crotonoyloxy)-1alpha-(2-methylbutyryloxy)-3,14-dehydro-Z-notonipetranone
Catalog No.:BCN7638
CAS No.:80514-14-3
A new monoterpene glucoside and complete assignments of dihydroflavonols of Pulicaria jaubertii: potential cytotoxic and blood pressure lowering activity.[Pubmed:26247309]
Nat Prod Res. 2016 Jun;30(11):1280-8.
One new monoterpene glucoside and five dihydroflavonols were isolated for the first time from the aerial parts of Pulicaria jaubertii and identified as p-menthane-2-O-beta-D-glucopyranoside [1], dihydroquercetin (taxifolin) [2], 7,3'-di-O-methyltaxifolin [3], 3'-O-methyltaxifolin [4], 7-O-methyltaxifolin (Padmatin) [5] and 7-O-methyl-dihydrokampferol (7-O-methylaromadenderin) [6]. The structures of these compounds were unambiguously assigned on the basis of NMR spectroscopic data ((1)H, (13)C, DEPT, HSQC, HMBC) and MS analysis. 2D-NMR methods required revision of assignments of H-6 and H-8 for dihydroflavonol compounds. Possible cytotoxic activity as well as blood pressure (BP) lowering activity were tested. The alcoholic extract showed cytotoxic activity against prostate carcinoma (PC-3), breast carcinoma (MCF-7) and hepatocellular carcinoma (HepG-2) human cell lines with IC50 19.1, 20.0 and 24.1 mug, respectively. The higher dose levels of the alcoholic extract significantly reduced normal BP of rats in a dose-dependent manner.
Antifungal activity of Heterothalamus alienus metabolites.[Pubmed:18386258]
Phytother Res. 2008 Apr;22(4):524-8.
The chemical study of Heterothalamus alienus gave rutin, spathulenol (1), (1R,7S)-germacra-4(15),5,10(14)-trien-1beta-ol (2), sakuranetin (3), Padmatin 3-acetate (4), (2R,3R)-dihydroquercetin-7,3',4'-trimethyl ether (5), (2R,3R)-dihydroquercetin-7,4'-dimethyl ether (6), (2R,3R)-3-acetoxy-5,7,4'-trihydroxyflavanone (7), as the main components of an antifungal extract of the aerial parts of the plant. Compound 2 showed moderate activity, with Epidermophyton floccosum being the most susceptible species (MIC = 100 microg/mL); compound 3 showed the best antifungal behavior having a broad spectrum of action and the lowest MICs. This flavanone along with flavanolol 5 showed very good activity against standardized (MIC = 31.2 microg/mL) as well as clinical isolates of Trichophyton rubrum and T. mentagrophytes (MIC ranges 31.2-62.5 microg/mL and 31.2-125 microg/mL, respectively) and demonstrated not only fungistatic but also fungicide properties. Flavanolol 6 was active against all the dermatophytes tested with MICs of 62.5-250 microg/mL. Rutin, spathulenol (1) and the 3-acetylated flavanones 4 and 7 were inactive or marginally active against the fungal panel.


