PHTPPCAS# 805239-56-9 |
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- AKT inhibitor VIII
Catalog No.:BCC1334
CAS No.:612847-09-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 805239-56-9 | SDF | Download SDF |
| PubChem ID | 11201035 | Appearance | Powder |
| Formula | C20H11F6N3O | M.Wt | 423.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 25 mg/mL (59.06 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
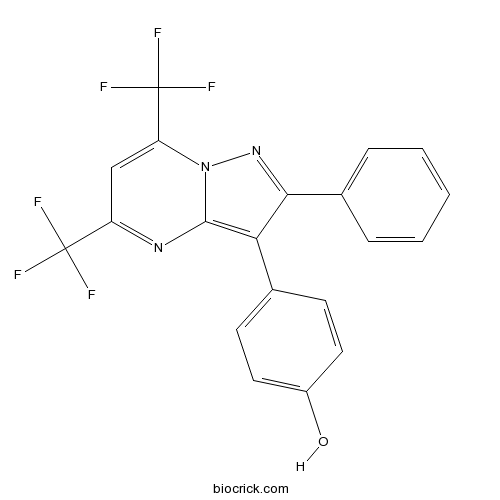
| Chemical Name | 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol | ||
| SMILES | C1=CC=C(C=C1)C2=NN3C(=CC(=NC3=C2C4=CC=C(C=C4)O)C(F)(F)F)C(F)(F)F | ||
| Standard InChIKey | AEZPAUSGTAHLOQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H11F6N3O/c21-19(22,23)14-10-15(20(24,25)26)29-18(27-14)16(11-6-8-13(30)9-7-11)17(28-29)12-4-2-1-3-5-12/h1-10,30H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective estrogen ERβ receptor antagonist that displays 36-fold selectivity over ERα. Exhibits full antagonism at ERβ in a cotransfection assay in human endometrial cancer cells (HEC-1), with minimal effects on ERα. |

PHTPP Dilution Calculator

PHTPP Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3623 mL | 11.8117 mL | 23.6233 mL | 47.2467 mL | 59.0584 mL |
| 5 mM | 0.4725 mL | 2.3623 mL | 4.7247 mL | 9.4493 mL | 11.8117 mL |
| 10 mM | 0.2362 mL | 1.1812 mL | 2.3623 mL | 4.7247 mL | 5.9058 mL |
| 50 mM | 0.0472 mL | 0.2362 mL | 0.4725 mL | 0.9449 mL | 1.1812 mL |
| 100 mM | 0.0236 mL | 0.1181 mL | 0.2362 mL | 0.4725 mL | 0.5906 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7beta-(3-Ethyl-cis-crotonoyloxy)-1alpha-(2-methylbutyryloxy)-3,14-dehydro-Z-notonipetranone
Catalog No.:BCN7638
CAS No.:80514-14-3
- Cis-N-Feruloyltyramine
Catalog No.:BCN3729
CAS No.:80510-09-4
- Grossamide
Catalog No.:BCN4571
CAS No.:80510-06-1
- Euchrestaflavanone A
Catalog No.:BCN3576
CAS No.:80510-05-0
- Veratrine
Catalog No.:BCN8444
CAS No.:8051-02-3
- Glaucocalyxin B
Catalog No.:BCN8440
CAS No.:80508-81-2
- Tenacigenin B
Catalog No.:BCN4342
CAS No.:80508-42-5
- Mezlocillin Sodium Monohydrate
Catalog No.:BCC5634
CAS No.:80495-46-1
- Rubifolic acid
Catalog No.:BCN4341
CAS No.:80489-65-2
- Fluticasone propionate
Catalog No.:BCC4907
CAS No.:80474-14-2
- Sapogenins Glycosides
Catalog No.:BCC5320
CAS No.:8047-15-2
- Paeoniflorigenone
Catalog No.:BCN3933
CAS No.:80454-42-8
- Vitexin 2''-O-(4'''-O-acetyl)rhamnoside
Catalog No.:BCN6740
CAS No.:80537-98-0
- Schinifoline
Catalog No.:BCN4343
CAS No.:80554-58-1
- Grifolic acid
Catalog No.:BCN4344
CAS No.:80557-12-6
- Balsalazide
Catalog No.:BCC5225
CAS No.:80573-04-2
- Vitamin B6
Catalog No.:BCN8345
CAS No.:8059-24-3
- 2',5,6',7-Tetrahydroxyflavanone
Catalog No.:BCN4345
CAS No.:80604-16-6
- 2',5,6',7-Tetraacetoxyflavanone
Catalog No.:BCN4346
CAS No.:80604-17-7
- 8-Demethylsideroxylin
Catalog No.:BCN3117
CAS No.:80621-54-1
- Rifaximin (Xifaxan)
Catalog No.:BCC3848
CAS No.:80621-81-4
- Sanggenone C
Catalog No.:BCN6350
CAS No.:80651-76-9
- Fuziline
Catalog No.:BCN2822
CAS No.:80665-72-1
- Dihydroergotoxine mesylate
Catalog No.:BCC6671
CAS No.:8067-24-1
Hindbrain Estrogen Receptor Regulation of Ventromedial Hypothalamic Glycogen Metabolism and Glucoregulatory Transmitter Expression in the Hypoglycemic Male Rat.[Pubmed:30954669]
Neuroscience. 2019 Apr 4. pii: S0306-4522(19)30215-5.
Estrogen receptor-alpha (ERalpha) and -beta (ERbeta) occur in key elements of the brain gluco-homeostatic network in both sexes, including the hindbrain dorsal vagal complex (DVC), but the influence of distinct receptor populations on this critical function is unclear. The ventromedial hypothalamic nucleus (VMN) maintains glucose balance by integrating nutrient, endocrine, and neurochemical cues, including metabolic sensory information supplied by DVC A2 noradrenergic neurons. Current research utilized the selective ERalpha and ERbeta antagonists MPP and PHTPP to characterize effects of DVC ERs on VMN norepinephrine (NE) activity and metabolic neurotransmitter signaling in insulin-induced hypoglycemic (IIH) male rats. Data show that ERbeta inhibits VMN glycogen synthase and stimulates phosphorylase protein expression, while attenuating hypoglycemic augmentation of glycogen content. Furthermore, both ERs attenuate VMN glucose concentrations during IIH. Hypoglycemic up-regulation of nitric oxide (NO) and brain-derived neurotrophic factor (BDNF) signaling was correspondingly driven by ERalpha or -beta, whereas GABA and steroidogenic factor-1 were respectively suppressed independently of ER input or by ERbeta. IIH intensified VMN NE accumulation by ERbeta-dependent mechanisms, but did not alter NE levels in other gluco-regulatory loci. ERbeta amplified the magnitude of insulin-induced decline in blood glucose. Both ER regulate corticosterone, but not glucagon secretion during IIH and oppose hypoglycemic diminution of circulating free fatty acids. These findings identify distinguishing versus common VMN functions targeted by DVC ERalpha and -beta. Sex differences in hypoglycemic VMN NE accumulation, glycogen metabolism, and transmitter signaling may involve, in part, discrepant regulatory involvement or differential magnitude of impact of these hindbrain ERs.
Estrogen receptor beta suppresses inflammation and the progression of prostate cancer.[Pubmed:30864712]
Mol Med Rep. 2019 May;19(5):3555-3563.
Previous studies demonstrated that estrogen receptor beta (ERbeta) signaling alleviates systemic inflammation in animal models, and suggested that ERbetaselective agonists may deactivate microglia and suppress T cell activity via downregulation of nuclear factor kappalightchainenhancer of activated B cells (NFkappaB). In the present study, the role of ERbeta in lipopolysaccharide (LPS)induced inflammation and association with NFkappaB activity were investigated in PC3 and DU145 prostate cancer cell lines. Cells were treated with LPS to induce inflammation, and ELISA was performed to determine the expression levels of inflammatory cytokines, including tumor necrosis factoralpha (TNFalpha), monocyte chemoattractant protein 1 (MCP1), interleukin (IL)1beta and IL6. MTT and Transwell assays, and Annexin V/propidium iodide staining were conducted to measure cell viability, apoptosis and migration, respectively. Protein expression was determined via western blot analysis. LPSinduced inflammation resulted in elevated expression levels of TNFalpha, IL1beta, MCP1 and IL6 compared with controls. ERbeta overexpression significantly inhibited the LPSinduced production of TNFalpha, IL1beta, MCP1 and IL6. In addition, the results indicated that ERbeta suppressed viability and migration, and induced apoptosis in prostate cancer cells, which was further demonstrated by altered expression of proliferating cell nuclear antigen, Bcell lymphoma 2associated X protein, caspase3, Ecadherin and matrix metalloproteinase2. These effects were reversed by treatment with the ERbeta antagonist PHTPP or ERbetaspecific short interfering RNA. ERbeta overexpression reduced the expression levels of p65 and phosphorylated NFkappaB inhibitor alpha (IkappaBalpha), but not total IkappaBalpha expression in LPStreated cells. In conclusion, ERbeta suppressed the viability and migration of the PC3 and DU145 prostate cancer cell lines and induced apoptosis. Furthermore, it reduced inflammation and suppressed the activation of the NFkappaB pathway, suggesting that ERbeta may serve roles as an antiinflammatory and anticancer agent in prostate cancer.
Estrogen receptor beta plays a protective role in zearalenone-induced oxidative stress in normal prostate epithelial cells.[Pubmed:30738973]
Ecotoxicol Environ Saf. 2019 May 15;172:504-513.
Zearalenone (ZEA) - a fungal mycotoxin is reported to both cause the oxidative stress associated with death of cells as well as induction of the proliferation of cells, depending on its concentration and the type of cells. ZEA due to its structural similarity to naturally occurring estrogens is able to bind to estrogen receptors and triggers estrogen-associated signaling pathways. The aim of this study is to evaluate whether the induction of oxidative stress in normal epithelial prostate PNT1A cells is associated with estrogenic activity of ZEA. We observed that ZEA-induced oxidative stress in PNT1A cells is associated with a decrease in the oxidative stress defense enzymes expression, cell cycle arrest in G2/M cell cycle phase as well as the decreased migration of cells. The results also suggest that the observed effect might be associated with the nuclear factor kappa-light-chain-enhancer of activated B cells (NFkappaB)- hypoxia inducible factor 1 alpha (HIF-1alpha) signaling pathway. The usage of estrogen receptor beta (ERbeta) selective antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]-phenol PHTPP showed that ERbeta activity is able to decrease the ZEA-induced oxidative stress, but is not enough to counteract it, indicating that ZEA-induced oxidative stress is only partially associated with estrogenic activity of ZEA.
ERbeta modulates genistein's cisplatin-enhancing activities in breast cancer MDA-MB-231 cells via P53-independent pathway.[Pubmed:30737644]
Mol Cell Biochem. 2019 Feb 8. pii: 10.1007/s11010-019-03505-y.
As one of the typical food-derived phytoestrogens, genistein (GEN) could bind to estrogen receptor (ER) and was reported to be closely related to breast cancer. Our former research showed that GEN interfered with the anti-tumor effects of cisplatin (CIS) in breast cancer MCF-7 (ERalpha+/ERbeta-) cells. However, it is not clear whether ER expression pattern affects GEN's modulation on CIS's activity. In the present study, breast cancer ERbeta knockdown (ERbetaKD) MDA-MB-231 (ERalpha-/ERbeta+) cell model was established via ERbeta RNAi lentivirus infection. The role of ERbeta expression in GEN's bioeffects on cells' response to CIS was investigated and was further double-checked by pathway-specific inhibitor PHTPP. Consistent results were harvested through cell viability analysis, cell cycle distribution flow cytometry, TUNEL staining, and expression detection of key biomarkers, Bax, Bcl-2, P21, P53, and cleaved caspase-3. Compared with the control group, PHTPP-treated or ERbetaKD cells exhibited higher sensitivity to both GEN and CIS treatment. GEN and CIS showed synergistic effects only in ERbeta-deficient cells. This effect mainly resulted in G2 phase arresting and apoptosis induction with the upregulation of P21 and Bax/Bcl-2 protein level. Besides, P53 expression was strikingly suppressed in ERbeta-deficient cells. This indicated ERbeta pathway deficiency might enhance GEN-CIS bioactivity via the downregulation of P53. In summary, our data imply that daily intake of GEN-rich diet could collaborate with CIS anti-tumor treatment in ERalpha-/ERbeta- breast cancer cases. ERbeta pathway might be one of the potential targets which elicit GEN's positive effects in ERalpha- breast cancer patients.
Estradiol promotes EMT in endometriosis via MALAT1/miR200s sponge function.[Pubmed:30500775]
Reproduction. 2018 Nov 1. pii: REP-18-0424.R2.
Endometriosis is an estrogen-dependent benign gynecological disease that shares some common features of malignancy. Epithelial-mesenchymal transition (EMT) has been recognized as a core mechanism of endometriosis. MALAT1 is widely known as EMT promoter, while miR200 family members (miR200s) are considered as EMT inhibitors. Previous studies have reported that MALAT1 up-regulation and miR200s down-regulation are observed in endometriosis. MiR200c has been regarded as the strongest member of miR200s to interact with MALAT1. However, whether MALAT1/miR200c regulates EMT remains largely unclear. In this study, the roles of miR200s and MALAT1 in ectopic endometrium were investigated. Additionally, the effects of E2 on EMT and MALAT1/miR200s were examined in both EECs and Ishikawa cells. Notably, E2 could up-regulate MALAT1 and down-regulate miR200s expression levels, and induce EMT in EECs and Ishikawa cells. PHTPP, an ERbeta antagonist, could reverse the effect of E2. Overexpression of miR200c and knockdown of MALAT1 significantly inhibited E2-mediated EMT, suggesting that both miR200c and MALAT1 are involved in the E2-induced EMT process in endometriosis. In addition, a reciprocal inhibition was found between miR200s and MALAT1. Therefore, the role of MALAT1/miR200c in EMT is influenced by the presence of estrogen during endometriosis development.
Sex differences in forebrain estrogen receptor regulation of hypoglycemic patterns of counter-regulatory hormone secretion and ventromedial hypothalamic nucleus glucoregulatory neurotransmitter and astrocyte glycogen metabolic enzyme expression.[Pubmed:30396594]
Neuropeptides. 2018 Dec;72:65-74.
The female ventromedial hypothalamic nucleus (VMN) is a focal substrate for estradiol (E) regulation of energy balance, feeding, and body weight, but how E shapes VMN gluco-regulatory signaling in each sex is unclear. This study investigated the hypothesis that estrogen receptor-alpha (ERalpha) and/or -beta (ERbeta) control VMN signals that inhibit [gamma-aminobutyric acid] or stimulate [nitric oxide, steroidogenic factor-1 (SF-1)] counter-regulation in a sex-dependent manner. VMN nitrergic neurons monitor astrocyte fuel provision; here, we examined how these ER regulate astrocyte glycogen metabolic enzyme, monocarboxylate transporter, and adrenoreceptor protein responses to insulin-induced hypoglycemia (IIH) in each sex. Testes-intact male and E-replaced ovariectomized female rats were pretreated by intracerebroventricular ERalpha antagonist (MPP) or ERbeta antagonist (PHTPP) administration before IIH. Data implicate both ER in hypoglycemic inhibition of neuronal nitric oxide synthase protein in each sex and up-regulation of glutamate decarboxylase65/67 and SF-1 expression in females. ERalpha and -beta enhance astrocyte AMPK and glycogen synthase expression and inhibit glycogen phosphorylase in hypoglycemic females, while ERbeta suppresses the same proteins in males. Differential VMN astrocyte protein responses to IIH may partially reflect ERalpha and -beta augmentation of ERbeta and down-regulation of alpha1, alpha2, and beta1 adrenoreceptor proteins in females, versus ERbeta repression of GPER and alpha2 adrenoreceptor profiles in males. MPP or PHTPP pretreatment blunted counter-regulatory hormone secretion in hypoglycemic males only, suggesting that in males one or more VMN neurotransmitters exhibiting sensitivity to forebrain ER may passively regulate this endocrine outflow, whereas female forebrain ERalpha and -beta are apparently uninvolved in these contra-regulatory responses.
Estrogen rescues heart failure through estrogen receptor Beta activation.[Pubmed:30376877]
Biol Sex Differ. 2018 Oct 30;9(1):48.
BACKGROUND: Recently, we showed that exogenous treatment with estrogen (E2) rescues pre-existing advanced heart failure (HF) in mice. Since most of the biological actions of E2 are mediated through the classical estrogen receptors alpha (ERalpha) and/or beta (ERbeta), and both these receptors are present in the heart, we examined the role of ERalpha and ERbeta in the rescue action of E2 against HF. METHODS: Severe HF was induced in male mice by transverse aortic constriction-induced pressure overload. Once the ejection fraction (EF) reached ~ 35%, mice were treated with selective agonists for ERalpha (PPT, 850 mug/kg/day), ERbeta (DPN, 850 mug/kg/day), or E2 (30 mug/kg/day) together with an ERbeta-antagonist (PHTPP, 850 mug/kg/day) for 10 days. RESULTS: EF of HF mice was significantly improved to 45.3 +/- 2.1% with diarylpropionitrile (DPN) treatment, but not with PPT (31.1 +/- 2.3%). E2 failed to rescue HF in the presence of PHTPP, as there was no significant improvement in the EF at the end of the 10-day treatment (32.5 +/- 5.2%). The improvement of heart function in HF mice treated with ERbeta agonist DPN was also associated with reduced cardiac fibrosis and increased cardiac angiogenesis, while the ERalpha agonist PPT had no significant effect on either cardiac fibrosis or angiogenesis. Furthermore, DPN improved hemodynamic parameters in HF mice, whereas PPT had no significant effect. CONCLUSIONS: E2 treatment rescues pre-existing severe HF mainly through ERbeta. Rescue of HF by ERbeta activation is also associated with stimulation of cardiac angiogenesis, suppression of fibrosis, and restoration of hemodynamic parameters.
17beta-Estradiol Enhances Schwann Cell Differentiation via the ERbeta-ERK1/2 Signaling Pathway and Promotes Remyelination in Injured Sciatic Nerves.[Pubmed:30356713]
Front Pharmacol. 2018 Oct 9;9:1026.
Remyelination is critical for nerve regeneration. However, the molecular mechanism involved in remyelination is poorly understood. To explore the roles of 17beta-estradiol (E2) for myelination in the peripheral nervous system, we used a co-culture model of rat dorsal root ganglion (DRG) explants and Schwann cells (SCs) and a regeneration model of the crushed sciatic nerves in ovariectomized (OVX) and non-ovariectomized (non-OVX) rats for in vitro and in vivo analysis. E2 promoted myelination by facilitating the differentiation of SCs in vitro, which could be inhibited by the estrogen receptors (ER) antagonist ICI182780, ERbeta antagonist PHTPP, or ERK1/2 antagonist PD98059. This suggests that E2 accelerates SC differentiation via the ERbeta-ERK1/2 signaling. Furthermore, E2 promotes remyelination in crushed sciatic nerves of both OVX and non-OVX rats. Interestingly, E2 also significantly increased the expression of the lysosome membrane proteins LAMP1 and myelin protein P0 in the regenerating nerves. Moreover, P0 has higher degree of colocalization with LAMP1 in the regenerating nerves. Taking together, our results suggest that E2 enhances Schwann cell differentiation and further myelination via the ERbeta-ERK1/2 signaling and that E2 increases the expression of myelin proteins and lysosomes in SCs to promotes remyelination in regenerating sciatic nerves.
17beta-Estradiol reduces inflammation and modulates antioxidant enzymes in colonic epithelial cells.[Pubmed:30336658]
Korean J Intern Med. 2018 Oct 22. pii: kjim.2018.098.
Background/Aims: Estrogen is known to have protective effect in colorectal cancer development. The aims of this study are to investigate whether estradiol treatment reduces inflammation in CCD841CoN, a female human colonic epithelial cell line and to uncover underlying mechanisms of estradiol effects. Methods: 17beta-Estradiol (E2) effect was measured by Western blot after inducing inf lammation of CCD841CoN by tumor necrosis factor alpha (TNF-alpha). Expression levels of estrogen receptor alpha (ERalpha) and beta (ERbeta), cyclooxygenase-2 (COX-2), nuclear factor-kappaB (NF-kappaB), heme oxygenase-1 (HO-1), and NAD(P)H-quinone oxidoreductase-1 (NQO-1) were also evaluated. Results: E2 treatment induced expression of ERbeta but did not increase that of ERalpha. E2 treatment for 48 hours significantly elevated the expression of anti-oxidant enzymes, HO-1 and NQO-1. TNF-alpha treatment significantly increased the level of activated NF-kappaB (p < 0.05), and this increase was significantly suppressed by treatment of 10 nM of E2 (p < 0.05). E2 treatment ameliorated TNF-alpha-induced COX-2 expression and decrease of HO-1 expression. 4-(2-phenyl-5,7-bis(trifluoromethyl) pyrazolo(1,5-a)pyrimidin-3-yl)phenol (PHTPP), antagonist of ERbeta, removed the inhibitory effect of E2 in the TNF-alpha-induced COX-2 expression (p = 0.05). Conclusions: Estrogen seems to inhibit inflammation in female human colonic epithelial cell lines, through down-regulation of NF-kappaB and COX-2 expression and induction of anti-oxidant enzymes such as HO-1 and NQO-1.
17beta-Estradiol/extrogen receptor beta alleviates apoptosis and enhances matrix biosynthesis of nucleus pulposus cells through regulating oxidative damage under a high glucose condition.[Pubmed:30257311]
Biomed Pharmacother. 2018 Nov;107:1004-1009.
BACKGROUND: Hyperglycemia in the Diabetes mellitus (DM) patients is a potential etiology of disc degeneration. 17beta-estradiol (17beta-E2) supplementation plays an anti-diabetic role in DM patients. OBJECTIVE: This study was aimed to investigate the role and mechanism of 17beta-E2 in regulating nucleus pulposus (NP) cell apoptosis and NP matrix production under a high glucose condition. METHODS: Rat NP cells were cultured in medium with a high glucose concentration (0.2M). 17beta-E2 was added into the culture medium to investigate its protective effects. The ERbeta inhibitor PHTPP and ERbeta activator ERB041 were used to investigate the effects of ERbeta. NP cell apoptosis was analyzed by flow cytometry and expression of apoptosis-related molecules. NP matrix production was evaluated by expression of matrix macromolecules. Additionally, intracellular reactive oxygen species (ROS) content was also detected. RESULTS: Compared with the control NP cells, 17beta-E2 decreased NP cell apoptosis ratio, down-regulated gene expression of Bax and caspase-3, up-regulated gene expression of Bcl-2, increased protein expression of cleaved PARP and cleaved caspase-3, and increased expression of matrix molecules (aggrecan and collagen II). Moreover, 17beta-E2 decreased ROS content. Further analysis showed that ERbeta inhibition partly reversed these effects of 17beta-E2 whereas ERbeta activation further promoted its effects. CONCLUSION: 17beta-E2/ERbeta interaction attenuates apoptosis and promotes matrix biosynthesis of NP cells through alleviating oxidative damage under a high glucose condition. This study provides new knowledge on strategies for retarding disc degeneration.
GPER and ERalpha mediate estradiol enhancement of mitochondrial function in inflamed adipocytes through a PKA dependent mechanism.[Pubmed:30253224]
J Steroid Biochem Mol Biol. 2019 Jan;185:256-267.
Obesity is associated with inflammation, dysregulated adipokine secretion, and disrupted adipose tissue mitochondrial function. Estradiol (E2) has been previously reported to increase mitochondrial function and biogenesis in several cell lines, but neither the type of oestrogen receptor (ERalpha, ERbeta and GPER) involved nor the mechanism whereby such effects are exerted have been fully described. Considering the anti-inflammatory activity of E2 as well as its effects in enhancing mitochondrial biogenesis, the aim of this study was to investigate the contribution of ERalpha, ERbeta, and GPER signaling to the E2-mediated enhancement of adipocyte mitochondrial function in a pro-inflammatory situation. 3T3-L1 cells were treated for 24 h with ER agonists (PPT, DPN, and G1) and antagonists (MPP, PHTPP, and G15) in the presence or absence of interleukin 6 (IL6), as a pro-inflammatory stimulus. Inflammation, mitochondrial function and biogenesis markers were analyzed. To confirm the involvement of the PKA pathway, cells were treated with a GPER agonist, a PKA inhibitor, and IL6. Mitochondrial function markers were analyzed. Our results showed that activation of ERalpha and GPER, but not ERbeta, was able to counteract the proinflammatory effects of IL6 treatment, as well as mitochondrial biogenesis and function indicators. Inhibition of PKA prevented the E2- and G1-associated increase in mitochondrial function markers. In conclusion E2 prevents IL6 induced inflammation in adipocytes and promotes mitochondrial function through the combined activation of both GPER and ERalpha. These findings expand our understanding of ER interactions under inflammatory conditions in female rodent white adipose tissue.
Alterations in human neutrophil function caused by bisphenol A.[Pubmed:30088793]
Am J Physiol Cell Physiol. 2018 Nov 1;315(5):C636-C642.
Bisphenol A (BPA) is a synthetic, organic compound frequently present in consumer plastics, including plastic-lined cans, water bottles, toys, and teeth sutures. Previous studies have shown that BPA can produce adverse health effects that include defects in reproductive function and altered prenatal/childhood development. However, little is known regarding the effects of BPA on immune function. In this study, we assessed the effect of BPA on human neutrophils, a critical component of the innate immune system's defense against pathogens. We found that BPA induces a concentration-dependent increase in reactive oxygen species (ROS) generation by neutrophils, which is inhibited by the estrogen receptor-beta antagonist PHTPP. Furthermore, incubation with the membrane-permeable calcium chelator BAPTA-AM and/or removal of extracellular calcium inhibited BPA-induced ROS production, indicating that the process is calcium dependent. Transwell chemotaxis assays revealed that BPA exposure reduces the chemotactic capacity of neutrophils in a gradient of the bacterial cell wall component f-Met-Leu-Phe, a potent chemoattractant. Exposure to BPA also inhibits the ability of neutrophils to kill methicillin-resistant Staphylococcus aureus, a leading human pathogen. Our findings reveal that BPA alters the in vitro function of neutrophils, including ROS production, chemotaxis, and bacterial killing, and raises the possibility of altered innate immunity in vivo, especially in those with compromised immune function and who can be exposed to BPA in a wide variety of products.
Impact of Perfluorooctane Sulfonate on Reproductive Ability of Female Mice through Suppression of Estrogen Receptor alpha-Activated Kisspeptin Neurons.[Pubmed:29939337]
Toxicol Sci. 2018 Oct 1;165(2):475-486.
Perfluorooctane sulfonate (PFOS) is used extensively in industrial and household applications. High exposure to PFOS has been associated with increased odds of irregular and long menstrual cycles in women. However, the underlying mechanisms remain to be elucidated. Herein, we show that adult female mice appeared prolongation of diestrus and reduction of corpora luteum within a week of oral administration of PFOS (10 mg/kg), which are associated with decreases in the levels of serum progesterone, LH and hypothalamic GnRH. The number of AVPV-kisspeptin neurons and the AVPV-kisspeptin expression were increased in proestrus mice or OVX-mice treated with high-dose estradiol benzoate (0.05 mg/kg), which were suppressed by the administration of PFOS. The administration of PFOS or GPR54 antagonist P234 prevented the generation of LH-surge in OVX-mice treated with high-dose E2. In hypothalamic slices incubated in 100 nM E2 for 4 h, the AVPV-kisspeptin expression was significantly enhanced, which was inhibited by PFOS in a dose-dependent manner or estrogen receptor alpha (ERalpha) antagonist MPP, but not ERbeta antagonist PHTPP. The incubation of ERalpha agonist PPT rather than ERbeta agonist DPN could increase the level of AVPV-kisspeptin expression, which was sensitive to the treatment with PFOS. The administration of GPR54 agonist kisspeptin-10 in PFOS-mice could correct the prolongation of diestrus and reduction of corpora luteum, and recover the LH-surge and the levels of LH and GnRH. The results indicate that exposure to PFOS suppressed ERalpha-induced activation of AVPV-kisspeptin neurons leads to diestrus prolongation and ovulation reduction.
Effects of the Catechol and Methoxy Metabolites of 17beta-Estradiol on Nitric Oxide Production by Ovine Uterine Artery Endothelial Cells.[Pubmed:29929429]
Reprod Sci. 2019 Apr;26(4):459-468.
Nitric oxide (NO) production is essential to facilitate rises in uterine blood flow (UBF) during pregnancy. It has been proposed that the metabolites of E2beta, 2-hydroxyestradiol (2-OHE2), 4-hydroxyestradiol (4-OHE2), 2-methoxyestradiol (2-ME2), and 4-methoxyestradiol (4-ME2) play a role in mediating vasodilation and rises in UBF during pregnancy. We previously showed that the E2beta metabolites stimulate prostacyclin production in pregnancy-derived ovine uterine artery endothelial cells (P-UAECs); however, it is unknown whether the E2beta metabolites also induce NO production. Herein, UAECs derived from nonpregnant and pregnant ewes were used to test the hypothesis that E2beta metabolites stimulate NO production in a pregnancy-specific manner. Specific estrogen receptor (ER) and adrenergic receptor (AR) antagonists were used to determine the roles of ERs or ARs in E2beta metabolite-induced NO production. E2beta and its metabolites increased total nitric oxide metabolites (NOx) levels (NO2 + NO3) in P-UAECs, but not in NP-UAECs. Pretreatment with combined 1 micromol/L 1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP; ER-alpha antagonist) and 1 micromol/L 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP; ER-beta antagonist) inhibited the rises in NOx levels stimulated by E2beta and 2-ME2, but had no effect on 2-OHE2-, 4-OHE2-, or 4-ME2-stimulated rises in NOx levels. Pretreatment with yohimbine (alpha2-AR antagonist) and propranolol (beta2,3-AR antagonist) inhibited the rises in NOx levels stimulated by 2-OHE2, but not by E2beta, 4-OHE2, 2-ME2, or 4-ME2. These data demonstrate that E2beta metabolites stimulate NO synthesis via ERs or ARs in UAECs in a pregnancy-specific manner, suggesting that these metabolites contribute to rises in vasodilation and UBF during pregnancy.
Pyrazolo[1,5-a]pyrimidines: estrogen receptor ligands possessing estrogen receptor beta antagonist activity.[Pubmed:15537344]
J Med Chem. 2004 Nov 18;47(24):5872-93.
In our search for novel subtype-selective estrogen receptor (ER) ligands, we have examined various heterocyclic units as core structural elements. Here, we have investigated the fused, bicyclic pyrazolo[1,5-a]pyrimidine core, which is a system that allows for analogues to be readily assembled in a library-like fashion. This series of pyrazolo[1,5-a]pyrimidine ER ligands provided us with a new pharmacological profile for an ER ligand: compounds that are passive on both ERs, with a distinct potency selectivity in favor of ERbeta. The most distinctive ligand in this series, 2-phenyl-3-(4-hydroxyphenyl)-5,7-bis(trifluoromethyl)-pyrazolo[1,5-a]pyrimidine, was 36-fold selective for ERbeta in binding. Curiously, on the basis of molecular modeling, the ERbeta binding selectivity of compounds in this series appears to be derived from differing orientations that they adapt in the ligand binding pockets of ERalpha vs ERbeta. In transcription assays this pyrazolopyrimidine was fully effective as an ERbeta antagonist while exhibiting no significant activity on ERalpha. Thus, this ligand functions as a potency- and efficacy-selective ERbeta antagonist that would abrogate estrogen action through ERbeta with minimal effects on its activity through ERalpha; as such, it could be used to study the biological function of ERbeta.


