Oroxylin A 7-O-beta-D-glucuronideCAS# 36948-76-2 |
Quality Control & MSDS
Number of papers citing our products

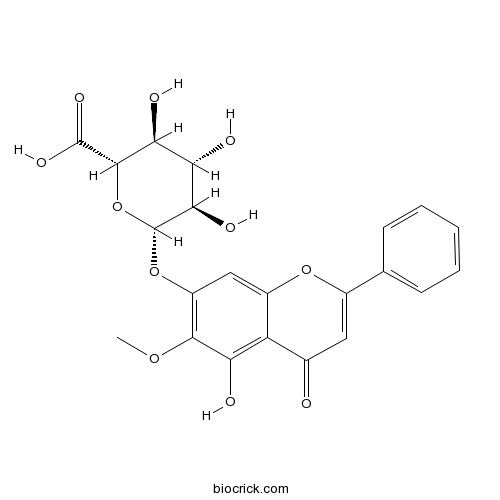
Chemical structure

3D structure
| Cas No. | 36948-76-2 | SDF | Download SDF |
| PubChem ID | 14655552 | Appearance | Yellow powder |
| Formula | C22H20O11 | M.Wt | 460.39 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 5,7-Dihydroxy 6-methoxyflavone 7-glucuronide; 6-Methoxybaicalein 7-glucuronide; Oroxyloside | ||
| Solubility | Soluble in methan | ||
| Chemical Name | (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-(5-hydroxy-6-methoxy-4-oxo-2-phenylchromen-7-yl)oxyoxane-2-carboxylic acid | ||
| SMILES | COC1=C(C=C2C(=C1O)C(=O)C=C(O2)C3=CC=CC=C3)OC4C(C(C(C(O4)C(=O)O)O)O)O | ||
| Standard InChIKey | QXIPXNZUEQYPLZ-QSUZLTIMSA-N | ||
| Standard InChI | InChI=1S/C22H20O11/c1-30-19-13(32-22-18(27)16(25)17(26)20(33-22)21(28)29)8-12-14(15(19)24)10(23)7-11(31-12)9-5-3-2-4-6-9/h2-8,16-18,20,22,24-27H,1H3,(H,28,29)/t16-,17-,18+,20-,22+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Oroxylin A-7-O-glucuronide and wogonoside should also be served as the chemical markers together with baicalin for the quality control of herbs and proprietary traditional Chinese medicine (PTCM) products of radix Scutellariae.. Oroxylin A 7-O-glucuronide can inhibit total prolyl oligopeptidase (POP) activity. |
| Targets | IL Receptor | Prolyl oligopeptidase |
| In vitro | Flavonoids from Scutellaria baicalensis and their bioactivities.[Pubmed: 19829679]Beijing Da Xue Xue Bao. 2009 Oct 18;41(5):578-84.To study the flavonoids of Scutellaria baicalensis and their bioactivities.
Flavonoids with prolyl oligopeptidase inhibitory activity isolated from Scutellaria racemosa Pers.[Pubmed: 20117183 ]Fitoterapia. 2010 Sep;81(6):552-6.Prolyl oligopeptidase (POP) is a serine protease highly expressed in the brain that hydrolyses peptide bonds at the carboxyl terminal of prolyl residues. There is evidence that this enzyme participates in several functions of the central nervous system. Scutellaria racemosa Pers demonstrated significant and selective POP inhibition.
|
| Structure Identification | J Pharm Biomed Anal. 2009 Oct 15;50(3):298-306.Contents of major bioactive flavones in proprietary traditional Chinese medicine products and reference herb of radix Scutellariae.[Pubmed: 19481403 ]
Plos One, 2013, 8(11):e80197.Molecular modeling reveals the novel inhibition mechanism and binding mode of three natural compounds to staphylococcal α-hemolysin.[Reference: WebLink]
|

Oroxylin A 7-O-beta-D-glucuronide Dilution Calculator

Oroxylin A 7-O-beta-D-glucuronide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1721 mL | 10.8604 mL | 21.7207 mL | 43.4414 mL | 54.3018 mL |
| 5 mM | 0.4344 mL | 2.1721 mL | 4.3441 mL | 8.6883 mL | 10.8604 mL |
| 10 mM | 0.2172 mL | 1.086 mL | 2.1721 mL | 4.3441 mL | 5.4302 mL |
| 50 mM | 0.0434 mL | 0.2172 mL | 0.4344 mL | 0.8688 mL | 1.086 mL |
| 100 mM | 0.0217 mL | 0.1086 mL | 0.2172 mL | 0.4344 mL | 0.543 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Icilin
Catalog No.:BCC4074
CAS No.:36945-98-9
- Hydramicromelin B
Catalog No.:BCN7560
CAS No.:369391-55-9
- 6-epi-Augustifolin
Catalog No.:BCN3233
CAS No.:369390-94-3
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- p-Coumaryl alcohol
Catalog No.:BCN3922
CAS No.:3690-05-9
- TC 14012
Catalog No.:BCC7910
CAS No.:368874-34-4
- Aucuparin
Catalog No.:BCN7450
CAS No.:3687-28-3
- Tramiprosate
Catalog No.:BCC7727
CAS No.:3687-18-1
- Meclofenoxate hydrochloride
Catalog No.:BCC4170
CAS No.:3685-84-5
- 1,5-Pentanediol diacrylate
Catalog No.:BCC8426
CAS No.:36840-85-4
- Naringenin triacetate
Catalog No.:BCN5425
CAS No.:3682-04-0
- Isohemiphloin
Catalog No.:BCN5424
CAS No.:3682-02-8
- TMPyP4 tosylate
Catalog No.:BCC7899
CAS No.:36951-72-1
- FCCP
Catalog No.:BCC5659
CAS No.:370-86-5
- Zinterol hydrochloride
Catalog No.:BCC6911
CAS No.:37000-20-7
- (-)-Variabilin
Catalog No.:BCN4815
CAS No.:370102-93-5
- Carbetocin
Catalog No.:BCC6304
CAS No.:37025-55-1
- Cyclo(L-Phe-L-Pro)
Catalog No.:BCN4029
CAS No.:3705-26-8
- Cyclo(Gly-L-Pro)
Catalog No.:BCN4059
CAS No.:3705-27-9
- Z-Glu-OBzl
Catalog No.:BCC2780
CAS No.:3705-42-8
- Procyanidin C1
Catalog No.:BCN6317
CAS No.:37064-30-5
- Azlocillin sodium salt
Catalog No.:BCC4763
CAS No.:37091-65-9
- IC-87114
Catalog No.:BCC1161
CAS No.:371242-69-2
- Murrangatin
Catalog No.:BCN5426
CAS No.:37126-91-3
[Flavonoids from Scutellaria baicalensis and their bioactivities].[Pubmed:19829679]
Beijing Da Xue Xue Bao Yi Xue Ban. 2009 Oct 18;41(5):578-84.
OBJECTIVE: To study the flavonoids of Scutellaria baicalensis and their bioactivities. METHODS: Chromatographic methods were used to separate compounds, spectral methods were used to determine the structures and bioactivities of the flavonoids were screened in several models in vitro. RESULTS: Nine compounds were isolated and identified as baicalein (1), wogonin (2), oroxylin A (3), 5, 7, 2', 6'-tetrahydroxyflavone (4), viscidulin III (5), baicalin (6), wogonoside (7), oroxylin A-7-O-beta-D-glucuronide (8) and chrysin-6-C-alpha-L-arabinopyranosyl-8-C-beta-D-glucopyranoside (9). CONCLUSION: Baicalein had good anti-bacteria activity, and some compounds showed inhibiting activity against IL-1beta converting enzyme. The 13C NMR data of compounds 9 were assigned correctly by 2D nuclear magnetic resonance (NMR).
Contents of major bioactive flavones in proprietary traditional Chinese medicine products and reference herb of radix Scutellariae.[Pubmed:19481403]
J Pharm Biomed Anal. 2009 Oct 15;50(3):298-306.
A simple and efficient HPLC/UV method for the simultaneous determination of six bioactive flavones, namely baicalein, baicalin, wogonin, wogonoside, oroxylin A and oroxylin A-7-O-glucuronide, has been developed and applied for their content determination in reference herb and proprietary traditional Chinese medicine (PTCM) products of radix Scutellariae. The chromatographic separation was carried out on a Thermo C(18) column and linear gradient elution was employed with a mobile phase containing acetonitrile and 20 mM sodium dihydrogen phosphate buffer (pH 4.6). All the analytes were detected by PDA detector at a wavelength of 270 nm. Contents of the analytes in radix Scutellariae containing PTCM products in forms of capsule, soft capsule, tablet and dripping pill and the reference herb of radix Scutellariae were analyzed by sonicator extraction with methanol and water mixture (80:20) containing 1 mM HCl for 30 min followed by HPLC analysis. Separation of the six analytes was achieved within 25 min with good linearity (R(2)>0.99). The R.S.D. of both the intra-day and inter-day precision for all the six analytes was below 10.14%. The accuracy at different concentrations was within the range of -7.83 to 4.06%. The extraction recovery was within the range of 89.22-107.33% for all the analytes. Contents of the six flavones were found to vary significantly among different products with glycosides, such as baicalin, wogonoside and oroxylin A-7-O-glucuronide, in much greater quantity than their corresponding aglycones. In addition to baicalin (18.54+/-0.71%, w/w), the commonly used marker compound for radix Scutellariae, wogonoside (3.54+/-0.18%, w/w) and oroxylin A-7-O-glucuronide (2.84+/-0.14%, w/w) also existed in abundant amount in the reference herb. Our findings suggested that wogonoside and oroxylin A-7-O-glucuronide should also be served as the chemical markers together with baicalin for the quality control of herbs and PTCM products of radix Scutellariae.
Flavonoids with prolyl oligopeptidase inhibitory activity isolated from Scutellaria racemosa Pers.[Pubmed:20117183]
Fitoterapia. 2010 Sep;81(6):552-6.
Prolyl oligopeptidase (POP) is a serine protease highly expressed in the brain that hydrolyses peptide bonds at the carboxyl terminal of prolyl residues. There is evidence that this enzyme participates in several functions of the central nervous system. Scutellaria racemosa Pers demonstrated significant and selective POP inhibition. Fractionation of the hydroalcoholic extract resulted in the isolation of four main constituents identified for the first time from S. racemosa Pers, the triterpenoid lupeol (1) and the flavonoids oroxylin A (5,7-dihydroxy-6-methoxyflavone, 2), hispidulin (4',5,7-trihydroxy-6-methoxyflavone, 3), and oroxyloside (oroxylin A 7-O-glucuronide, 4). Inhibitory assays indicated that 3 and 4 at a concentration of 100 microM inhibit 43 and 34% of total POP activity, respectively.


